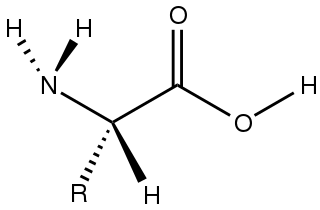
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code.

The Miller–Urey experiment (or Miller experiment) is a famous chemistry experiment that simulated the conditions thought at the time (1952) to be present in the atmosphere of the early, prebiotic Earth, in order to test the hypothesis of the chemical origin of life under those conditions. The experiment used water (H2O), methane (CH4), ammonia (NH3), hydrogen (H2), and an electric arc (the latter simulating hypothesized lightning).
Peptides are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
Proteinoids, or thermal proteins, are protein-like, often cross-linked molecules formed abiotically from amino acids. Sidney W. Fox initially proposed that they may have been precursors to the first living cells (protocells). The term was also used in the 1960s to describe peptides that are shorter than twenty amino acids found in hydrolysed protein, but this term is no longer commonly used.

Stanley Lloyd Miller was an American chemist who made landmark experiments in the origin of life by demonstrating that a wide range of vital organic compounds can be synthesized by fairly simple chemical processes from inorganic substances. In 1952 he carried out the Miller–Urey experiment, which showed that complex organic molecules could be synthesised from inorganic precursors. The experiment was widely reported, and provided support for the idea that the chemical evolution of the early Earth had led to the natural synthesis of chemical building blocks of life from inanimate inorganic molecules. He has been described as the "father of prebiotic chemistry".
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer. The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers.
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.
Native Chemical Ligation (NCL) is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most effective method for synthesizing native or modified proteins of typical size.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.

Polyphosphazenes include a wide range of hybrid inorganic-organic polymers with a number of different skeletal architectures with the backbone P-N-P-N-P-N-. In nearly all of these materials two organic side groups are attached to each phosphorus center. Linear polymers have the formula (N=PR1R2)n, where R1 and R2 are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which small phosphazene rings are connected together by organic chain units. Other architectures are available, such as block copolymer, star, dendritic, or comb-type structures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group of Harry R. Allcock.
Friedrich Hermann Leuchs was a German chemist.
The Juliá–Colonna epoxidation is an asymmetric poly-leucine catalyzed nucleophilic epoxidation of electron deficient olefins in a triphasic system. The reaction was reported by Sebastian Juliá at the Chemical Institute of Sarriá in 1980, with further elaboration by both Juliá and Stefano Colonna.
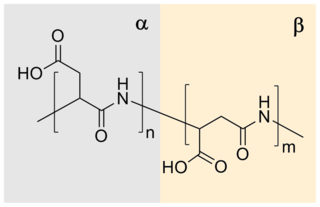
Polyaspartic acid (PASA) is a biodegradable, water-soluble condensation polymer based on the amino acid aspartic acid. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogels. The resulting hydrogels are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.

A sequence-controlled polymer is a macromolecule, in which the sequence of monomers is controlled to some degree. This control can be absolute but not necessarily. In other words, a sequence-controlled polymer can be uniform or non-uniform (Ð>1). For example, an alternating copolymer synthesized by radical polymerization is a sequence-controlled polymer, even if it is also a non-uniform polymer, in which chains have different chain-lengths and slightly different compositions. A biopolymer with a perfectly-defined primary structure is also a sequence-controlled polymer. However, in the case of uniform macromolecules, the term sequence-defined polymer can also be used.

Diaminomaleonitrile (DAMN) is an organic compound composed of two amino groups and two nitrile groups bonded to a central alkene unit. The systematic name reflects its relationship to maleic acid.
Formamide-based prebiotic chemistry is a reconstruction of the beginnings of life on Earth, assuming that formamide could accumulate in sufficiently high amounts to serve as the building block and reaction medium for the synthesis of the first biogenic molecules.

The Bailey peptide synthesis is a name reaction in organic chemistry developed 1949 by J. L. Bailey. It is a method for the synthesis of a peptide from α-amino acid-N-carboxylic acid anhydrides (NCAs) and amino acids or peptide esters. The reaction is characterized by short reaction times and a high yield of the target peptide.
Jessica R. Kramer is an American biomedical engineer working as an Assistant Professor of Bio-engineering and Adjunct Assistant Professor of Pharmaceutics and Pharmaceutical Chemistry at the University of Utah. Kramer’s research lab focuses on the synthesis and application of glycopolypeptides.
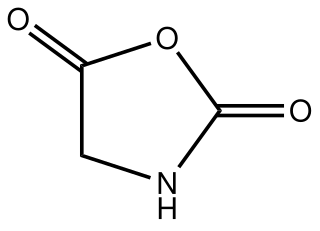
Glycine N-carboxyanhydride is an organic compound with the formula HNCH(CO)2O. A colorless solid, it is the product of the phosgenation of glycine. Glycine N-carboxyanhydride is the simplest member of the amino acid N-carboxyanhydrides. It is also the parent of the 2,5-Oxazolidinedione family of heterocycles.














