Related Research Articles

Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

In organic chemistry, phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2 and structure HO(O)C−C6H4−C(O)OH. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale. Phthalic acid is one of three isomers of benzenedicarboxylic acid, the others being isophthalic acid and terephthalic acid.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. Haloarenes are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.

Isoquinoline is an individual chemical specimen - a heterocyclic aromatic organic compound - as well as the name of a family of many thousands of natural plant alkaloids, any one of which might be referred to as "an isoquinoline". It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in many naturally occurring alkaloids such as papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.

Decalin, a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives.

Fluorene, or 9H-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. Despite its name, it does not contain the element fluorine, but rather it comes from the violet fluorescence it exhibits. For commercial purposes it is obtained from coal tar. It is insoluble in water and soluble in many organic solvents. Although sometimes classified as a polycyclic aromatic hydrocarbon, the five-membered ring has no aromatic properties. Fluorene is mildly acidic.

Naphthalenesulfonates are derivatives of sulfonic acid which contain a naphthalene functional unit. A related family of compounds are the aminonaphthalenesulfonic acids. Of commercial importance are the alkylnaphthalene sulfonates, which are used as superplasticizers in concrete. They are produced on a large scale by condensation of naphthalenesulfonate or alkylnaphthalenesulfonates with formaldehyde.

2-Naphthylamine is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, but it is a known carcinogen and has largely been replaced by less toxic compounds.
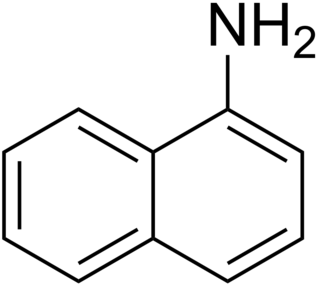
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer. It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.

Armstrong's acid (naphthalene-1,5-disulfonic acid) is a fluorescent organic compound with the formula C10H6(SO3H)2. It is one of several isomers of naphthalenedisulfonic acid. It a colorless solid, typically obtained as the tetrahydrate. Like other sulfonic acids, it is a strong acid. It is named for British chemist Henry Edward Armstrong.

1,8-Diaminonaphthalene is an organic compound with the formula C10H6(NH2)2. It is one of several isomeric naphthalenediamines. It is a colorless solid that darkens in air due to oxidation. It is a precursor to commercial pigments.
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs), a family of compounds with many uses.

In organic chemistry, peri-naphthalenes are particular derivatives of naphthalene with the formula C10H6-1,8-X2.

1-Bromonaphthalene is an organic compound with the formula C10H7Br.

3-Hydroxy-2-naphthoic acid is an organic compound with the formula C10H6(OH)(CO2H). It is one of the several hydroxynaphthoic acids. It is a precursor to some azo dyes and pigments. It is prepared by carboxylation of 2-naphthol by the Kolbe–Schmitt reaction.

Naphthalene-2-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the mono- and trihydrates C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being naphthalene-1-sulfonic acid. The compound is mainly used in the production of dyes via nitration en route to aminonaphthalenesulfonic acids. The compound is prepared by sulfonation of naphthalene with sulfuric acid, however under equilibrating conditions that allow the 1-sulfonic acid isomer to convert to the more stable 2-sulfonic acid.
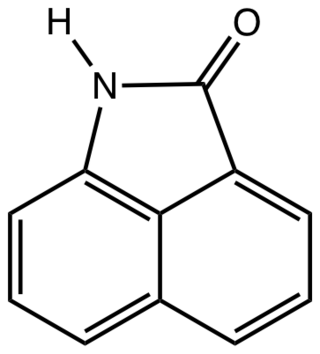
Naphtholactam is an organic compound derived from naphthalene. It is a tricyclic species consisting of a naphthalene core fused with a lactam (NH-CO-) at the 1,8-positions. The N-alkyl derivatives are commercially important.
References
- ↑ Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi : 10.1002/14356007.a17_009.