
Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs.

In organic chemistry, phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2 and structure HO(O)C−C6H4−C(O)OH. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale. Phthalic acid is one of three isomers of benzenedicarboxylic acid, the others being isophthalic acid and terephthalic acid.
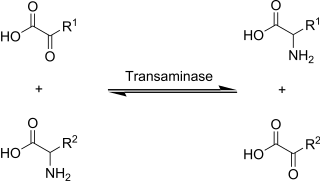
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids.This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.

2-Naphthylamine or 2-aminonaphthalene is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, but it is a known carcinogen and has largely been replaced by less toxic compounds.

Armstrong's acid (naphthalene-1,5-disulfonic acid) is a fluorescent organic compound with the formula C10H6(SO3H)2. It is one of several isomers of naphthalenedisulfonic acid. It a colorless solid, typically obtained as the tetrahydrate. Like other sulfonic acids, it is a strong acid. It is named for British chemist Henry Edward Armstrong.

Dansyl chloride or 5-(dimethylamino)naphthalene-1-sulfonyl chloride is a reagent that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green–fluorescent sulfonamide adducts. It can also be made to react with secondary amines. Dansyl chloride is widely used to modify amino acids; specifically, protein sequencing and amino acid analysis. Dansyl chloride may also be denoted DNSC. Likewise, a similar derivative, dansyl amide is known as DNSA.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.

Pipecolic acid (piperidine-2-carboxylic acid) is an organic compound with the formula HNC5H9CO2H. It is a carboxylic acid derivative of piperidine and, as such, an amino acid, although not one encoded genetically. Like many other α-amino acids, pipecolic acid is chiral, although the S-stereoisomer is more common. It is a colorless solid.
1-Naphthol, or α-naphthol, is an organic compound with the formula C10H7OH. It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds.
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs), a family of compounds with many uses.

2,6-Naphthalenedicarboxylic acid is an organic compound with the formula C10H6(CO2H)2. This colorless solid is one of several isomers of naphthalenedicarboxylic acid. It is a precursor to the high performance polyester polyethylene naphthalate (PEN, poly(ethylene-2,6-naphthalene dicarboxylate)). It is also used in the synthesis of some metal-organic frameworks.
Aminonaphthalenesulfonic acids are compounds with the composition C10H6(NH2)(SO3H), being derived from naphthalene (C10H8) substituted by an amino and sulfonic acid groups. These compounds are colorless solids. They are useful precursors to dyes.

Tobias acid (2-amino-1-naphthalenesulfonic acid) is an organic compound with the formula C10H6(SO3H)(NH2). It is named after the German chemist Georg Tobias. It is one of several aminonaphthalenesulfonic acids, which are derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear otherwise. It is used in the synthesis of azo dyes such as C.I. Acid Yellow 19 and C.I. Pigment Red 49. It is prepared via the Bucherer reaction of 2-hydroxynaphthalene-1-sulfonic acid with ammonia and ammonium sulfite.

In organic chemistry, peri-naphthalenes are particular derivatives of naphthalene with the formula C10H6-1,8-X2.

3-Hydroxy-2-naphthoic acid is an organic compound with the formula C10H6(OH)(CO2H). It is one of the several hydroxynaphthoic acids. It is a precursor to some azo dyes and pigments. It is prepared by carboxylation of 2-naphthol by the Kolbe–Schmitt reaction.

2-Carboxybenzaldehyde is a chemical compound. It consists of a benzene ring, with an aldehyde and a carboxylic acid as substituents that are ortho to each other. The compound exhibits ring–chain tautomerism: the two substituents can react with each other to form 3-hydroxyphthalide, a cyclic lactol. This lactol reacts readily with Grignard reagents, forming alkyl- and aryl-substituted phthalides. Other benzo-fused heterocyclic compounds can be derived from 2-carboxybenzaldehyde, including isoindolinones and phthalazinones, with a variety of pharmacological properties, such as the antihistamine azelastine.

Naphthalene-1-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the dihydrate C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being the more stable naphthalene-2-sulfonic acid. The compound is mainly used in the production of dyes.

Naphthalene-2-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the mono- and trihydrates C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being naphthalene-1-sulfonic acid. The compound is mainly used in the production of dyes via nitration en route to aminonaphthalenesulfonic acids. The compound is prepared by sulfonation of naphthalene with sulfuric acid, however under equilibrating conditions that allow the 1-sulfonic acid isomer to convert to the more stable 2-sulfonic acid.
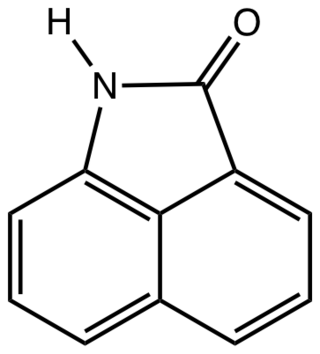
Naphtholactam is an organic compound derived from naphthalene. It is a tricyclic species consisting of a naphthalene core fused with a lactam (NH-CO-) at the 1,8-positions. The N-alkyl derivatives are commercially important.
















