Related Research Articles

Structural biology is a branch of molecular biology, biochemistry, and biophysics concerned with the molecular structure of biological macromolecules, how they acquire the structures they have, and how alterations in their structures affect their function. This subject is of great interest to biologists because macromolecules carry out most of the functions of cells, and it is only by coiling into specific three-dimensional shapes that they are able to perform these functions. This architecture, the "tertiary structure" of molecules, depends in a complicated way on each molecule's basic composition, or "primary structure."

Protein folding is the physical process by which a protein chain acquires its native three-dimensional structure, a conformation that is usually biologically functional, in an expeditious and reproducible manner. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from a random coil. Each protein exists as an unfolded polypeptide or random coil when translated from a sequence of mRNA to a linear chain of amino acids. This polypeptide lacks any stable (long-lasting) three-dimensional structure. As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure.

A detergent is a surfactant or a mixture of surfactants with cleansing properties in dilute solutions. These substances are usually alkylbenzene sulfonates, a family of compounds that are similar to soap but are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

Surfactants are molecules that spontaneously bond with each other to form sealed bubbles. Surfactants are compounds that lower the surface tension between two liquids, between a gas and a liquid, or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants.

A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents.

Peripheral membrane proteins are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.
A chaotropic agent is a molecule in water solution that can disrupt the hydrogen bonding network between water molecules. This has an effect on the stability of the native state of other molecules in the solution, mainly macromolecules by weakening the hydrophobic effect. For example, a chaotropic agent reduces the amount of order in the structure of a protein formed by water molecules, both in the bulk and the hydration shells around hydrophobic amino acids, and may cause its denaturation.

The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity.
Aqueous biphasic systems (ABS) or aqueous two-phase systems (ATPS) are clean alternatives for traditional organic-water solvent extraction systems.

An amphiphile is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. This forms the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism. Organic compounds containing hydrophilic groups at both ends of a prolate molecule are called bolaamphiphilic. Common amphiphilic substances are soaps, detergents, and lipoproteins.
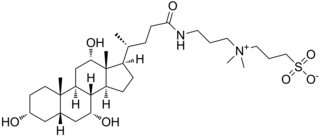
CHAPS is a zwitterionic surfactant used in the laboratory to solubilize biological macromolecules such as proteins. It may be synthesized from cholic acid and is zwitterionic due to its quaternary ammonium and sulfonate groups; it is structurally similar to certain bile acids, such as taurodeoxycholic acid and taurochenodeoxycholic acid. It is used as a non-denaturing detergent in the process of protein purification and is especially useful in purifying membrane proteins, which are often sparingly soluble or insoluble in aqueous solution due to their native hydrophobicity.
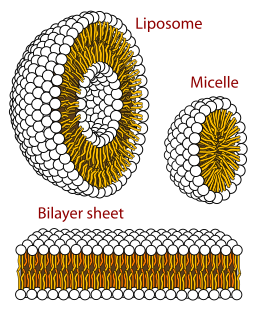
Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.

Water-in-water (W/W) emulsion is a system that consists of droplets of water-solvated molecules in another continuous aqueous solution; both the droplet and continuous phases contain different molecules that are entirely water-soluble. As such, when two entirely aqueous solutions containing different water-soluble molecules are mixed, water droplets containing predominantly one component are dispersed in water solution containing another component. Recently, such a water-in-water emulsion was demonstrated to exist and be stable from coalescence by the separation of different types of non-amphiphilic, but water-soluble molecular interactions. These molecular interactions include hydrogen bonding, pi stacking, and salt bridging. This w/w emulsion was generated when the different water-solvated molecular functional groups get segregated in an aqueous mixture consisting of polymer and liquid crystal molecules.
Octyl glucoside is a nonionic surfactant frequently used to solubilise integral membrane proteins for studies in biochemistry. Structurally, it is a glycoside derived from glucose and octanol. Like Genapol X-100 and Triton X-100, it is a nonphysiological amphiphile that makes lipid bilayers less "stiff".
A model lipid bilayer is any bilayer assembled in vitro, as opposed to the bilayer of natural cell membranes or covering various sub-cellular structures like the nucleus. They are used to study the fundamental properties of biological membranes in a simplified and well-controlled environment, and increasingly in bottom-up synthetic biology for the construction of artificial cells. A model bilayer can be made with either synthetic or natural lipids. The simplest model systems contain only a single pure synthetic lipid. More physiologically relevant model bilayers can be made with mixtures of several synthetic or natural lipids.

The term macromolecular assembly (MA) refers to massive chemical structures such as viruses and non-biologic nanoparticles, cellular organelles and membranes and ribosomes, etc. that are complex mixtures of polypeptide, polynucleotide, polysaccharide or other polymeric macromolecules. They are generally of more than one of these types, and the mixtures are defined spatially, and with regard to their underlying chemical composition and structure. Macromolecules are found in living and nonliving things, and are composed of many hundreds or thousands of atoms held together by covalent bonds; they are often characterized by repeating units. Assemblies of these can likewise be biologic or non-biologic, though the MA term is more commonly applied in biology, and the term supramolecular assembly is more often applied in non-biologic contexts. MAs of macromolecules are held in their defined forms by non-covalent intermolecular interactions, and can be in either non-repeating structures, or in repeating linear, circular, spiral, or other patterns. The process by which MAs are formed has been termed molecular self-assembly, a term especially applied in non-biologic contexts. A wide variety of physical/biophysical, chemical/biochemical, and computational methods exist for the study of MA; given the scale of MAs, efforts to elaborate their composition and structure and discern mechanisms underlying their functions are at the forefront of modern structure science.
Chemical chaperones are a class of small molecules that function to enhance the folding and/or stability of proteins. Chemical chaperones are a broad and diverse group of molecules, and they can influence protein stability and polypeptide organization through a variety of mechanisms. Chemical chaperones are used for a range of applications, from production of recombinant proteins to treatment of protein misfolding in vivo.

SDS-PAGE, is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel allows to eliminate the influence of structure and charge, and proteins are separated solely on the basis of differences in their molecular weight.
References
- ↑ Bowie, J (2001). "Stabilizing membrane proteins". Current Opinion in Structural Biology. 11 (4): 397–402. doi:10.1016/S0959-440X(00)00223-2. ISSN 0959-440X.
- ↑ Tribet, C; Audebert, R; Popot, JL (24 December 1996). "Amphipols: polymers that keep membrane proteins soluble in aqueous solutions". Proceedings of the National Academy of Sciences of the United States of America. 93 (26): 15047–50. Bibcode:1996PNAS...9315047T. doi:10.1073/pnas.93.26.15047. PMC 26353 . PMID 8986761.
- ↑ Popot, J.-L., et al. (2011) Amphipols from A to Z. Annu. Rev. Biophys.40:379-408.
- ↑ Zoonens, M., Popot, J.-L. (2014) Amphipols for each season. J. Membr. Biol.247:759-796.
- ↑ Popot, J.-L. (2018) Membrane proteins in aqueous solutions: From detergents to amphipols. Springer, New York, in the press.
- ↑ Pocanschi, C.L., Dahmane, T., Gohon, Y., Rappaport, F., Apell, H.-J., Kleinschmidt, J.H., Popot, J.-L. (2006) Amphipathic polymers: tools to fold integral membrane proteins to their active form. Biochemistry45:13954-13961.
- ↑ Dahmane, T., Damian, M., Mary, S., Popot, J.-L., Banères, J.-L. (2009) Amphipol-assisted in vitro folding of G protein-coupled receptors. Biochemistry48:6516-6521.
- ↑ Althoff, T., Mills, D.J., Popot, J.-L., Kühlbrandt, W. (2011) Assembly of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J.30:4652-4664.
- ↑ Liao, M., Cao, E., Julius, D., Cheng, Y. (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature504:107-112.
- ↑ Popot, J.-L. (2018) Membrane proteins in aqueous solutions. From detergents to amphipols. Springer, New York, xxv + 708 p.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |