Related Research Articles
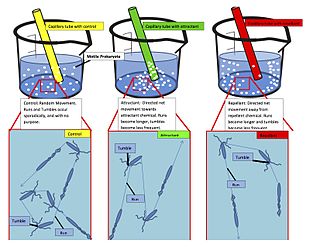
Chemotaxis is the movement of an organism or entity in response to a chemical stimulus. Somatic cells,bacteria,and other single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food by swimming toward the highest concentration of food molecules,or to flee from poisons. In multicellular organisms,chemotaxis is critical to early development and development as well as in normal function and health. In addition,it has been recognized that mechanisms that allow chemotaxis in animals can be subverted during cancer metastasis. The aberrant chemotaxis of leukocytes and lymphocytes also contribute to inflammatory diseases such as atherosclerosis,asthma,and arthritis. Sub-cellular components,such as the polarity patch generated by mating yeast,may also display chemotactic behavior.

Neutrophils are a type of white blood cell. More specifically,they form the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. They form an essential part of the innate immune system,with their functions varying in different animals.

Granulocytes are cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. Such granules distinguish them from the various agranulocytes. All myeloblastic granulocytes are polymorphonuclear. They have varying shapes (morphology) of the nucleus;and are referred to as polymorphonuclear leukocytes. In common terms,polymorphonuclear granulocyte refers specifically to "neutrophil granulocytes",the most abundant of the granulocytes;the other types have varying morphology. Granulocytes are produced via granulopoiesis in the bone marrow.

Chemokines,or chemotactic cytokines,are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes,as well as other cell types,including endothelial and epithelial cells. In addition to playing a major role in the activation of host immune responses,chemokines are important for biological processes,including morphogenesis and wound healing,as well as in the pathogenesis of diseases like cancers.

The Wisconsin Alumni Research Foundation is the independent nonprofit technology transfer organization serving the University of Wisconsin–Madison and Morgridge Institute for Research. It provides significant research support,granting tens of millions of dollars to the university each year and contributing to the university's "margin of excellence".

Interleukin 8 is a chemokine produced by macrophages and other cell types such as epithelial cells,airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage vesicles,the Weibel-Palade bodies. In humans,the interleukin-8 protein is encoded by the CXCL8 gene. IL-8 is initially produced as a precursor peptide of 99 amino acids which then undergoes cleavage to create several active IL-8 isoforms. In culture,a 72 amino acid peptide is the major form secreted by macrophages.

Transfusion-related acute lung injury (TRALI) is the serious complication of transfusion of blood products that is characterized by the rapid onset of excess fluid in the lungs. It can cause dangerous drops in the supply of oxygen to body tissues. Although changes in transfusion practices have reduced the incidence of TRALI,it was the leading cause of transfusion-related deaths in the United States from fiscal year 2008 through fiscal year 2012.
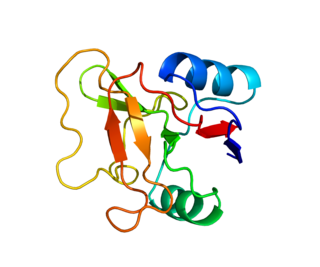
L-selectin,also known as CD62L,is a cell adhesion molecule found on the cell surface of leukocytes,and the blastocyst. It is coded for in the human by the SELL gene. L-selectin belongs to the selectin family of proteins,which recognize sialylated carbohydrate groups containing a Sialyl LewisX (sLeX) determinant. L-selectin plays an important role in both the innate and adaptive immune responses by facilitating leukocyte-endothelial cell adhesion events. These tethering interactions are essential for the trafficking of monocytes and neutrophils into inflamed tissue as well as the homing of lymphocytes to secondary lymphoid organs. L-selectin is also expressed by lymphoid primed hematopoietic stem cells and may participate in the migration of these stem cells to the primary lymphoid organs. In addition to its function in the immune response,L-selectin is expressed on embryonic cells and facilitates the attachment of the blastocyst to the endometrial endothelium during human embryo implantation.

Chemokine ligand 2 (CXCL2) is a small cytokine belonging to the CXC chemokine family that is also called macrophage inflammatory protein 2-alpha (MIP2-alpha),Growth-regulated protein beta (Gro-beta) and Gro oncogene-2 (Gro-2). CXCL2 is 90% identical in amino acid sequence as a related chemokine,CXCL1. This chemokine is secreted by monocytes and macrophages and is chemotactic for polymorphonuclear leukocytes and hematopoietic stem cells. The gene for CXCL2 is located on human chromosome 4 in a cluster of other CXC chemokines. CXCL2 mobilizes cells by interacting with a cell surface chemokine receptor called CXCR2.

Ralph Snyderman is Chancellor Emeritus at Duke University,James B. Duke Professor of Medicine,and Executive Director of the Duke Center for Personalized Health Care. He served as chancellor for health affairs and dean of the School of Medicine from 1989 to July 2004. Under his leadership,Duke University created the Duke University Health System (DUHS) to develop and operate a comprehensive health delivery system,and he was its founding President and Chief Executive Officer. DUHS,with its practice networks,ambulatory care centers,home health services,community hospitals,university hospital,and satellite collaborations demonstrated the power of academic medicine to deliver the best of care to broad communities. Snyderman helped lead the creation of the largest academic clinical research organization worldwide. During his tenure,Duke University Hospital was ranked 6th overall in the nation and its medical school ranked 4th. Snyderman is a leader in the conception and development of personalized health care,an evolving model of national health care delivery. He has articulated the need to move the current focus of health care from the treatment of disease-events to personalized,predictive,preventive,and participatory care that is focused on the patient. As Senior Vice-President at Genentech,he led the development of powerful new molecular biology therapeutics. Ralph Snyderman was the recipient of the 2012 David E. Rogers Award from the Association of American Medical Colleges which recognized him as "The Father of Personalized Medicine." He is a member of the Association of American Medical Colleges,Association of American Physicians,American Academy of Arts &Sciences,and the National Academy of Medicine.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response,involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

Leukocyte adhesion deficiency-1 (LAD1) is a rare and often fatal genetic disorder in humans.
Chemorepulsion is the directional movement of a cell away from a substance. Of the two directional varieties of chemotaxis,chemoattraction has been studied to a much greater extent. Only recently have the key components of the chemorepulsive pathway been elucidated. The exact mechanism is still being investigated,and its constituents are currently being explored as likely candidates for immunotherapies.
Uropods,in immunology,refer to the hind part of polarized cells during cell migration that stabilize and move the cell. Polarized leukocytes move using amoeboid cell migration mechanisms,with a small leading edge,main cell body,and posterior uropod protrusion. Cytoskeleton contraction and extension,controlled by various polarized signals,helps propel the cell body forward. Leukocyte polarization is an important requirement for migration,activation and apoptosis in the adaptive and innate immune systems;most leukocytes,including monocytes,granulocytes,and T and B lymphocytes migrate to and from primary and secondary lymphoid organs to tissues to initiate immune responses to pathogens.
Peter Richard Huttenlocher was a German-American pediatric neurologist and neuroscientist who discovered how the brain develops in children. He is considered to be one of the fathers of developmental cognitive neuroscience.

Dean Hamilton Kedes is an American scientist in the field of virology and current director of the medical scientist training program at the University of Virginia school of medicine.

John I. Gallin is an American medical researcher who has contributed to the understanding of innate immunity but especially chronic granulomatous disease,a phagocyte disorder. Gallin was appointed director of the NIH Clinical Center on May 1,1994,and served until January 8,2017. He serves as the chief scientific officer for the Clinical Center and associate director for clinical research at the National Institutes of Health.
Lisa Robinson is a clinician-scientist. She is a University of Toronto professor in the Department of Paediatrics and the Vice Dean Strategy and Operations at the Faculty of Medicine,former Head of the Division of Nephrology at The Hospital for Sick Children,a Senior Scientist at the SickKids Research Institute,and the first-ever Chief Diversity officer for the Faculty of Medicine at University of Toronto.
Marco Baggiolini is a Swiss immunologist and biochemist known for the discovery and the analysis of the first chemokines. Chemokines act as chemoattractants to guide the migration of cells. Some control cells of the immune system,some promote the growth of new blood vessels,some cause inflammation in response to bacterial infection and viruses,for example,to activate cells to initiate an immune response or promote wound healing.
Peter N. Devreotes is an American scientist and the Isaac Morris &Lucille Elizabeth Hay Professor and former director of the department of cell biology,with joint appointments in the Center for Cell Dynamics and department of biological chemistry at the Johns Hopkins University School of Medicine. He also serves on the scientific advisory board of the Allen Institute for Cell Science. He is best known for his contribution in the field of eukaryotic chemotaxis,signal transduction,and phosphoinositides biology.
References
- ↑ Easton, John (August 19, 2013). "Peter Huttenlocher, pediatric neurologist, 1931-2013". University of Chicago. Retrieved October 1, 2021.
- ↑ "Professor Anna Huttenlocher". Clare Hall, Cambridge . Retrieved October 1, 2021.
- 1 2 3 "Anna Huttenlocher". University of Wisconsin–Madison. Retrieved October 1, 2021.
- ↑ Lokuta, M. A., Nuzzi, P. A., & Huttenlocher, A. (2003). Calpain regulates neutrophil chemotaxis. Proceedings of the National Academy of Sciences of the United States of America, 100(7), 4006–4011. https://doi.org/10.1073/pnas.0636533100
- ↑ Franco, S. J., Rodgers, M. A., Perrin, B. J., Han, J., Bennin, D. A., Critchley, D. R., & Huttenlocher, A. (2004). Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature cell biology, 6(10), 977–983. https://doi.org/10.1038/ncb1175
- ↑ Mathias, J. R., Perrin, B. J., Liu, T. X., Kanki, J., Look, A. T., & Huttenlocher, A. (2006). Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of Leukocyte Biology, 80(6), 1281–1288. https://doi.org/10.1189/jlb.0506346
- ↑ de Oliveira, S., Rosowski, E. & Huttenlocher, A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16, 378–391 (2016). https://doi.org/10.1038/nri.2016.49
- ↑ Yoo, S. K., Starnes, T. W., Deng, Q., & Huttenlocher, A. (2011). Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature, 480(7375), 109–112. https://doi.org/10.1038/nature10632
- ↑ "Anna Huttenlocher, MD". American Society for Clinical Investigation . Retrieved October 1, 2021.
- ↑ "FACULTY PROMOTIONS AS OF MARCH 3, 2008". University of Wisconsin–Madison. May 1, 2008. Retrieved October 1, 2021.
- ↑ "DR. ANNA HUTTENLOCHER AWARDED GRADUATE SCHOOL'S H.I. ROMNES FELLOWSHIP AWARD". University of Wisconsin–Madison. May 1, 2008. Retrieved October 1, 2021.
- ↑ "ANNA HUTTENLOCHER, MD, RECEIVES THE BURROUGHS-WELLCOME FUND'S CLINICAL SCIENTIST AWARD". University of Wisconsin–Madison. May 1, 2008. Retrieved October 1, 2021.
- ↑ "DR. ANNA HUTTENLOCHER, MD, EARNS WARF KELLETT MID-CAREER AWARD". University of Wisconsin–Madison. January 1, 2011. Retrieved October 1, 2021.
- ↑ "ANNA HUTTENLOCHER, MD, ELECTED TO THE ASSOCIATION OF AMERICAN PHYSICIANS". University of Wisconsin–Madison. February 1, 2012. Retrieved October 1, 2021.
- ↑ "ANNA HUTTENLOCHER, MD, ELECTED TO NATIONAL ACADEMY OF MEDICINE". University of Wisconsin-Madison. October 1, 2015. Retrieved October 1, 2021.
- ↑ "ANNA HUTTENLOCHER, MD, AWARDED A UW2020 GRANT". University of Wisconsin-Madison. June 1, 2017. Retrieved October 1, 2021.
- ↑ "DR. ANNA HUTTENLOCHER ELECTED AS AN AMERICAN SOCIETY FOR CELL BIOLOGY FELLOW". University of Wisconsin–Madison. October 1, 2017. Retrieved October 1, 2021.
- ↑ "DR. HUTTENLOCHER RECEIVES WARF NAMED PROFESSORSHIP". University of Wisconsin–Madison. May 13, 2020. Retrieved October 1, 2021.
- ↑ "PEDIATRIC 'DREAM TEAM' MEMBERS AND OTHERS RECOGNIZED WITH SITC'S TEAM SCIENCE AWARD". University of Wisconsin–Madison. January 10, 2021. Retrieved October 1, 2021.
- ↑ "ANNA HUTTENLOCHER RECEIVES $3.3M NIH AWARD TO FURTHER CELL MIGRATION RESEARCH". University of Wisconsin–Madison. June 29, 2021. Retrieved October 1, 2021.
- ↑ "DR. ANNA HUTTENLOCHER AWARDED TWO-YEAR NIH-NIAID GRANT". University of Wisconsin–Madison. March 24, 2021. Retrieved October 1, 2021.
- ↑ Huttenlocher, Anna (2023). From Loss to Memory: Behind the Discovery of Synaptic Pruning. Cambridge, UK: Cambridge University Press. ISBN 9781009267052.