
Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in disease, called malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans, causing 405,000 deaths in 2018. It is also associated with the development of blood cancer and is classified as Group 2A carcinogen.
Glycophorin C plays a functionally important role in maintaining erythrocyte shape and regulating membrane material properties, possibly through its interaction with protein 4.1. Moreover, it has previously been shown that membranes deficient in protein 4.1 exhibit decreased content of glycophorin C. It is also an integral membrane protein of the erythrocyte and acts as the receptor for the Plasmodium falciparum protein PfEBP-2.

Duffy antigen/chemokine receptor (DARC), also known as Fy glycoprotein (FY) or CD234, is a protein that in humans is encoded by the ACKR1 gene.

Complement receptor type 1 (CR1) also known as C3b/C4b receptor or CD35 is a protein that in humans is encoded by the CR1 gene.

Plasmodium malariae is a parasitic protozoan that causes malaria in humans. It is one of several species of Plasmodium parasites that infect other organisms as pathogens, also including Plasmodium falciparum and Plasmodium vivax, responsible for most malarial infection. Found worldwide, it causes a so-called "benign malaria", not nearly as dangerous as that produced by P. falciparum or P. vivax. The signs include fevers that recur at approximately three-day intervals – a quartan fever or quartan malaria – longer than the two-day (tertian) intervals of the other malarial parasites.
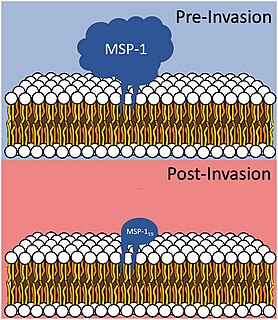
Merozoitesurface proteins are both integral and peripheral membrane proteins found on the surface of a merozoite, an early life cycle stage of a protozoan. Merozoite surface proteins, or MSPs, are important in understanding malaria, a disease caused by protozoans of the genus Plasmodium. During the asexual blood stage of its life cycle, the malaria parasite enters red blood cells to replicate itself, causing the classic symptoms of malaria. These surface protein complexes are involved in many interactions of the parasite with red blood cells and are therefore an important topic of study for scientists aiming to combat malaria.

Micronemes are secretory organelles, possessed by parasitic apicomplexans. Micronemes are located on the apical third of the protozoan body. They are surrounded by a typical unit membrane. On electron microscopy they have an electron-dense matrix due to the high protein content. They are specialized secretory organelles important for host-cell invasion and gliding motility.
Antigenic variation or antigenic alteration refers to the mechanism by which an infectious agent such as a protozoan, bacterium or virus alters the proteins or carbohydrates on its surface and thus avoids a host immune response. It is related to phase variation. Antigenic variation not only enables the pathogen to avoid the immune response in its current host, but also allows re-infection of previously infected hosts. Immunity to re-infection is based on recognition of the antigens carried by the pathogen, which are "remembered" by the acquired immune response. If the pathogen's dominant antigen can be altered, the pathogen can then evade the host's acquired immune system. Antigenic variation can occur by altering a variety of surface molecules including proteins and carbohydrates. Antigenic variation can result from gene conversion, site-specific DNA inversions, hypermutation, or recombination of sequence cassettes. The result is that even a clonal population of pathogens expresses a heterogeneous phenotype. Many of the proteins known to show antigenic or phase variation are related to virulence.
A blocking antibody is an antibody that does not have a reaction when combined with an antigen, but prevents other antibodies from combining with that antigen. This function of blocking antibodies has had a variety of clinical and experimental uses.
Basigin (BSG) also known as extracellular matrix metalloproteinase inducer (EMMPRIN) or cluster of differentiation 147 (CD147) is a protein that in humans is encoded by the BSG gene. This protein is a determinant for the Ok blood group system. There are three known antigens in the Ok system; the most common being Oka, OK2 and OK3. Basigin has been shown to be an essential receptor on red blood cells for the human malaria parasite, Plasmodium falciparum.

A malaria vaccine is a vaccine that is used to prevent malaria. The only approved vaccine as of 2015 is RTS,S, known by the trade name Mosquirix. It requires four injections, and has a relatively low efficacy. Due to this low efficacy, the World Health Organization (WHO) does not recommend the routine use of the RTS,S vaccine in babies between 6 and 12 weeks of age.

Glycophorin A , also known as GYPA, is a protein which in humans is encoded by the GYPA gene. GYPA has also recently been designated CD235a.
Human genetic resistance to malaria refers to inherited changes in the DNA of humans which increase resistance to malaria and result in increased survival of individuals with those genetic changes. The existence of these genotypes is likely due to evolutionary pressure exerted by parasites of the genus Plasmodium which cause malaria. Since malaria infects red blood cells, these genetic changes are most commonly alterations to molecules essential for red blood cell function, such as hemoglobin or other cellular proteins or enzymes of red blood cells. These alterations generally protect red blood cells from invasion by Plasmodium parasites or replication of parasites within the red blood cell.
CD36 antigen is a transmembrane, highly glycosylated, glycoprotein expressed by monocytes, macrophages, platelets, microvascular endothelial cells and adipose tissues. CD36 recognises oxidized low density lipoprotein, long chain fatty acids, anionic phospholipids, collagen types I, IV and V, thrombospondin and Plasmodium falciparum infected erythrocytes.

In molecular biology, Duffy binding proteins are found in plasmodia. Plasmodium vivax and Plasmodium knowlesi merozoites invade Homo sapiens erythrocytes that express Duffy blood group surface determinants. The Duffy receptor family is localised in micronemes, an organelle found in all organisms of the phylum Apicomplexa.
Russell J. Howard is an Australian-born scientist, CEO and entrepreneur. He was a pioneer in the fields of molecular parasitology, especially malaria, and in leading the commercialisation of one of the most important methods used widely today in molecular biology today called “DNA shuffling" or "Molecular breeding", a form of "Directed evolution".
Plasmodium coatneyi is a parasitic species that is an agent of malaria in nonhuman primates. P. coatneyi occurs in Southeast Asia. The natural host of this species is the rhesus macaque and crab-eating macaque, but there has been no evidence that zoonosis of P. coatneyi can occur through its vector, the female Anopheles mosquito.
KAHRP is a protein expressed by Plasmodium falciparum infecting erythrocytes. KAHRP is a major component of knobs, feature found on Plasmodium falciparum infected erythrocytes.
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a family of proteins present on the membrane surface of red blood cells that are infected by the malarial parasite Plasmodium falciparum. PfEMP1 is synthesized during the parasite's blood stage inside the RBC, during which the clinical symptoms of falciparum malaria are manifested. Acting as both an antigen and adhesion protein, it is thought to play a key role in the high level of virulence associated with P. falciparum. It was discovered in 1984 when it was reported that infected RBCs had unusually large-sized cell membrane proteins, and these proteins had antibody-binding (antigenic) properties. An elusive protein, its chemical structure and molecular properties were revealed only after a decade, in 1995. It is now established that there is not one but a large family of PfEMP1 proteins, genetically regulated (encoded) by a group of about 60 genes called var. Each P. falciparum is able to switch on and off specific var genes to produce a functionally different protein, thereby evading the host's immune system. RBCs carrying PfEMP1 on their surface stick to endothelial cells, which facilitates further binding with uninfected RBCs, ultimately helping the parasite to both spread to other RBCs as well as bringing about the fatal symptoms of P. falciparum malaria.











