Related Research Articles
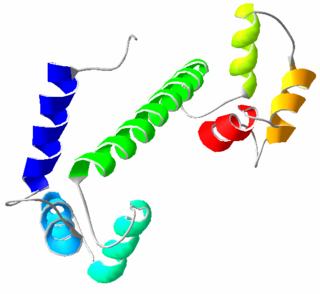
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

Hydrotropism is a plant's growth response in which the direction of growth is determined by a stimulus or gradient in water concentration. A common example is a plant root growing in humid air bending toward a higher relative humidity level.

Guard cells are specialized plant cells in the epidermis of leaves, stems and other organs that are used to control gas exchange. They are produced in pairs with a gap between them that forms a stomatal pore. The stomatal pores are largest when water is freely available and the guard cells become turgid, and closed when water availability is critically low and the guard cells become flaccid. Photosynthesis depends on the diffusion of carbon dioxide (CO2) from the air through the stomata into the mesophyll tissues. Oxygen (O2), produced as a byproduct of photosynthesis, exits the plant via the stomata. When the stomata are open, water is lost by evaporation and must be replaced via the transpiration stream, with water taken up by the roots. Plants must balance the amount of CO2 absorbed from the air with the water loss through the stomatal pores, and this is achieved by both active and passive control of guard cell turgor pressure and stomatal pore size.
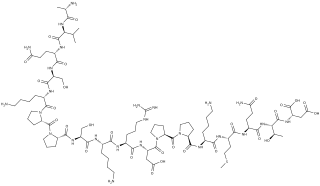
Systemin is a plant peptide hormone involved in the wound response in the family Solanaceae. It was the first plant hormone that was proven to be a peptide having been isolated from tomato leaves in 1991 by a group led by Clarence A. Ryan. Since then, other peptides with similar functions have been identified in tomato and outside of the Solanaceae. Hydroxyproline-rich glycopeptides were found in tobacco in 2001 and AtPeps were found in Arabidopsis thaliana in 2006. Their precursors are found both in the cytoplasm and cell walls of plant cells, upon insect damage, the precursors are processed to produce one or more mature peptides. The receptor for systemin was first thought to be the same as the brassinolide receptor but this is now uncertain. The signal transduction processes that occur after the peptides bind are similar to the cytokine-mediated inflammatory immune response in animals. Early experiments showed that systemin travelled around the plant after insects had damaged the plant, activating systemic acquired resistance, now it is thought that it increases the production of jasmonic acid causing the same result. The main function of systemins is to coordinate defensive responses against insect herbivores but they also affect plant development. Systemin induces the production of protease inhibitors which protect against insect herbivores, other peptides activate defensins and modify root growth. They have also been shown to affect plants' responses to salt stress and UV radiation. AtPEPs have been shown to affect resistance against oomycetes and may allow A. thaliana to distinguish between different pathogens. In Nicotiana attenuata, some of the peptides have stopped being involved in defensive roles and instead affect flower morphology.
Two-pore channels (TPCs) are eukaryotic intracellular voltage-gated and ligand gated cation selective ion channels. There are two known paralogs in the human genome, TPC1s and TPC2s. In humans, TPC1s are sodium selective and TPC2s conduct sodium ions, calcium ions and possibly hydrogen ions. Plant TPC1s are non-selective channels. Expression of TPCs are found in both plant vacuoles and animal acidic organelles. These organelles consist of endosomes and lysosomes. TPCs are formed from two transmembrane non-equivalent tandem Shaker-like, pore-forming subunits, dimerized to form quasi-tetramers. Quasi-tetramers appear very similar to tetramers, but are not quite the same. Some key roles of TPCs include calcium dependent responses in muscle contraction(s), hormone secretion, fertilization, and differentiation. Disorders linked to TPCs include membrane trafficking, Parkinson's disease, Ebola, and fatty liver.

Wall-associated kinases (WAKs) are one of many classes of plant proteins known to serve as a medium between the extracellular matrix (ECM) and cytoplasm of cell walls. They are serine-threonine kinases that contain epidermal growth factor (EGF) repeats, a cytoplasmic kinase and are located in the cell walls. They provide a linkage between the inner and outer surroundings of cell walls. WAKs are under a group of receptor-like kinases (RLK) that are actively involved in sensory and signal transduction pathways especially in response to foreign attacks by pathogens and in cell development. On the other hand, pectins are an abundant group of complex carbohydrates present in the primary cell wall that play roles in cell growth and development, protection, plant structure and water holding capacity.

In biology, phototropism is the growth of an organism in response to a light stimulus. Phototropism is most often observed in plants, but can also occur in other organisms such as fungi. The cells on the plant that are farthest from the light contain a hormone called auxin that reacts when phototropism occurs. This causes the plant to have elongated cells on the furthest side from the light. Phototropism is one of the many plant tropisms, or movements, which respond to external stimuli. Growth towards a light source is called positive phototropism, while growth away from light is called negative phototropism. Negative phototropism is not to be confused with skototropism, which is defined as the growth towards darkness, whereas negative phototropism can refer to either the growth away from a light source or towards the darkness. Most plant shoots exhibit positive phototropism, and rearrange their chloroplasts in the leaves to maximize photosynthetic energy and promote growth. Some vine shoot tips exhibit negative phototropism, which allows them to grow towards dark, solid objects and climb them. The combination of phototropism and gravitropism allow plants to grow in the correct direction.
PIN proteins are integral membrane proteins in plants that transport the anionic form of the hormone auxin across membranes. The discovery of the initial member of the PIN gene family, PIN1, occurred through the identification of the pin-formed1 (pin1) mutation in Arabidopsis thaliana. This mutation led to a stem that lacked almost all organs, including leaves and flowers.
Zhang Wenhao, also known as Wen-Hao Zhang, is a Chinese plant physiologist and nutritionist at the Institute of Botany, Chinese Academy of Sciences.
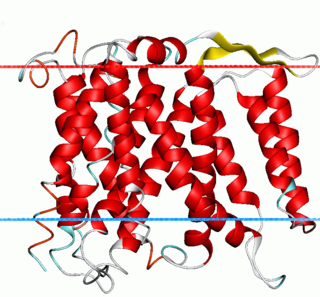
The Monovalent Cation:Proton Antiporter-1 (CPA1) Family (TC# 2.A.36) is a large family of proteins derived from Gram-positive and Gram-negative bacteria, blue-green bacteria, archaea, yeast, plants and animals. The CPA1 family belongs to the VIC superfamily. Transporters from eukaryotes have been functionally characterized to catalyze Na+:H+ exchange. Their primary physiological functions are thought to be in (1) cytoplasmic pH regulation, extruding the H+ generated during metabolism, and (2) salt tolerance (in plants), due to Na+ uptake into vacuoles. Bacterial homologues have also been found to facilitate Na+:H+ antiport, but some also catalyze Li+:H+ antiport or Ca2+:H+ antiport under certain conditions.

High Affinity K+ transporter HAK5 is a transport protein found on the cell surface membrane of plants under conditions of potassium deprivation. It is believed to act as a symporter for protons and the potassium ion, K+. Firstly discovered in barley, receiving the name of HvHAK1, it was soon after identified in the model plant Arabidopsis thaliana and named HAK5. These transporters belongs to the subgroup I of the KT-HAK-KUP family of plant proteins with obvious homology with both bacterial and fungal transport systems, which experienced a major diversification following land conquest. KT-HAK-KUP transporters are one of four different types of K+ transporter within the cell, but are unique as they do not have a putative pore forming domain like the other three; Shaker channels, KCO channels, HKT transporters. It is activated when the plant is situated in low soil with low potassium concentration, and has been shown to be located in higher concentration in the epidermis and vasculature of K+ deprived plants. By turning on, it increases the plants affinity (uptake) of potassium. Potassium plays a vital role in the plants growth, reproduction, immunity, ion homeostasis, and osmosis, which ensures the plants survival. It is the highest cationic molecule within the plant, accounting for 10% of the plants dry weight, which makes its uptake into the plant important. Each plant species has its own HAK5 transporter that is specific to that species and has different levels of affinity to K+. To operate and activate the HAK5 transporter, the external concentration of K+ must be lower than 10μM and up to 200μM. In Arabidopsis plants, when external potassium concentration is lower than 10μM, it is only HAK5 that is involved with the uptake of K+, then between 10 and 200μM both HAK5 and AKT1 are involved with the uptake of K+. HAK5 is coupled with CBL9/CIPK23 kinase's although the mechanism behind this has not yet been understood.
A cytokinin signaling and response regulator protein is a plant protein that is involved in a two step cytokinin signaling and response regulation pathway.
The P-type plasma membrane H+
-ATPase is found in plants and fungi. For the gastric H+
/K+
ATPase, see Hydrogen potassium ATPase.
Jian-Kang Zhu is a plant scientist, researcher and academic. He is a Senior Principal Investigator in the Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences (CAS). He is also the Academic Director of CAS Center of Excellence in Plant Sciences.

Ethylene (CH
2=CH
2) is an unsaturated hydrocarbon gas (alkene) acting as a naturally occurring plant hormone. It is the simplest alkene gas and is the first gas known to act as hormone. It acts at trace levels throughout the life of the plant by stimulating or regulating the ripening of fruit, the opening of flowers, the abscission (or shedding) of leaves and, in aquatic and semi-aquatic species, promoting the 'escape' from submergence by means of rapid elongation of stems or leaves. This escape response is particularly important in rice farming. Commercial fruit-ripening rooms use "catalytic generators" to make ethylene gas from a liquid supply of ethanol. Typically, a gassing level of 500 to 2,000 ppm is used, for 24 to 48 hours. Care must be taken to control carbon dioxide levels in ripening rooms when gassing, as high temperature ripening (20 °C; 68 °F) has been seen to produce CO2 levels of 10% in 24 hours.
Hydraulic signals in plants are detected as changes in the organism's water potential that are caused by environmental stress like drought or wounding. The cohesion and tension properties of water allow for these water potential changes to be transmitted throughout the plant.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.

Calcium signaling in Arabidopsis is a calcium mediated signalling pathway that Arabidopsis plants use in order to respond to a stimuli. In this pathway, Ca2+ works as a long range communication ion, allowing for rapid communication throughout the plant. Systemic changes in metabolites such as glucose and sucrose takes a few minutes after the stimulus, but gene transcription occurs within seconds. Because hormones, peptides and RNA travel through the vascular system at lower speeds than the plants response to wounds, indicates that Ca2+ must be involved in the rapid signal propagation. Instead of local communication to nearby cells and tissues, Ca2+ uses mass flow within the vascular system to help with rapid transport throughout the plant. Ca2+ moving through the xylem and phloem acts through a “calcium signature” receptor system in cells where they integrate the signal and respond with the activation of defense genes. These calcium signatures encode information about the stimulus allowing the response of the plant to cater towards the type of stimulus.
Christoph Benning is a German–American plant biologist. He is an MSU Foundation Professor and University Distinguished Professor at Michigan State University. Benning's research into lipid metabolism in plants, algae and photosynthetic bacteria, led him to be named Editor-in-Chief of The Plant Journal in October 2008.
The chlorophyll a/b-binding protein gene, otherwise known as the CAB gene, is one of the most thoroughly characterized clock-regulated genes in plants. There are a variety of CAB proteins that are derived from this gene family. Studies on Arabidopsis plants have shed light on the mechanisms of biological clocks under the regulation of CAB genes. Dr. Steve Kay discovered that CAB was regulated by a circadian clock, which switched the gene on in the morning and off in the late afternoon. The genes code for proteins that associate with chlorophyll and xanthophylls. This association aids the absorption of sunlight, which transfers energy to photosystem II to drive photosynthetic electron transport.
References
- 1 2 Hasegawa, Paul M.; Bressan, Ray A.; Zhu, Jian-Kang; Bohnert, Hans J. (2000). "Plant cellular and molecular responses to high salinity". Annual Review of Plant Physiology and Plant Molecular Biology. 51: 463–499. doi:10.1146/annurev.arplant.51.1.463. PMID 15012199.
- 1 2 "About Arabidopsis thaliana". unPAK. Retrieved 2018-05-14.
- 1 2 3 4 5 Ji, Hongtao (March 2013). "The Salt Overly Sensitive (SOS) Pathway: Established and Emerging Roles". Molecular Plant. 6 (2): 275–286. doi: 10.1093/mp/sst017 . PMID 23355543.
- ↑ Boursiac, Y.; Chen, S.; Luu, D. T.; Sorieul, M.; Van Den Dries, N.; Maurel, C. (2005). "Early Effects of Salinity on Water Transport in Arabidopsis Roots. Molecular and Cellular Features of Aquaporin Expression". Plant Physiology. 139 (2): 790–805. doi:10.1104/pp.105.065029. PMC 1255996 . PMID 16183846.
- ↑ Tester, M. (2003). "Na+ Tolerance and Na+ Transport in Higher Plants". Annals of Botany. 91 (5): 503–527. doi:10.1093/aob/mcg058. PMC 4242248 . PMID 12646496.
- 1 2 Yu, Lijuan; Nie, Jianing; Cao, Chunyan; Jin, Yakang; Yan, Min; Wang, Fuzheng; Liu, Ji; Xiao, Yun; Liang, Yongheng; Zhang, Wenhua (2010). "Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana". New Phytologist. 188 (3): 762–773. doi: 10.1111/j.1469-8137.2010.03422.x . PMID 20796215.
- ↑ Du, W.; Lin, H.; Chen, S.; Wu, Y.; Zhang, J.; Fuglsang, A. T.; Palmgren, M. G.; Wu, W.; Guo, Y. (2011). "Phosphorylation of SOS3-Like Calcium-Binding Proteins by Their Interacting SOS2-Like Protein Kinases is a Common Regulatory Mechanism in Arabidopsis". Plant Physiology. 156 (4): 2235–2243. doi:10.1104/pp.111.173377. PMC 3149935 . PMID 21685179.
- ↑ Liu, J. (1998). "A Calcium Sensor Homolog Required for Plant Salt Tolerance". Science. 280 (5371): 1943–1945. Bibcode:1998Sci...280.1943L. doi:10.1126/science.280.5371.1943. PMID 9632394.
- ↑ Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. (2000). "The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter". Proceedings of the National Academy of Sciences. 97 (12): 6896–6901. Bibcode:2000PNAS...97.6896S. doi: 10.1073/pnas.120170197 . PMC 18772 . PMID 10823923.
- 1 2 Shabala, Lana; Cuin, Tracey A.; Newman, Ian A.; Shabala, Sergey (2005). "Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants". Planta. 222 (6): 1041–1050. Bibcode:2005Plant.222.1041S. doi:10.1007/s00425-005-0074-2. PMID 16079998. S2CID 337766.
- ↑ Demidchik, V.; Tester, M. (2002). "Sodium Fluxes through Nonselective Cation Channels in the Plasma Membrane of Protoplasts from Arabidopsis Roots". Plant Physiology. 128 (2): 379–387. doi:10.1104/pp.010524. PMC 148901 . PMID 11842142.
- 1 2 Babourina, O. (2000). "Effect of Sudden Salt Stress on Ion Fluxes in Intact Wheat Suspension Cells". Annals of Botany. 85 (6): 759–767. doi:10.1006/anbo.2000.1136.