Related Research Articles
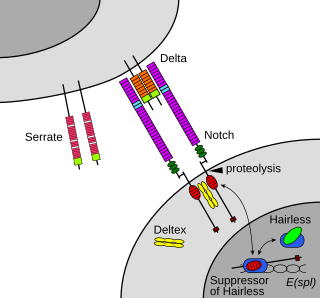
The Notch signaling pathway is a highly conserved cell signaling system present in most animals. Mammals possess four different notch receptors, referred to as NOTCH1, NOTCH2, NOTCH3, and NOTCH4. The notch receptor is a single-pass transmembrane receptor protein. It is a hetero-oligomer composed of a large extracellular portion, which associates in a calcium-dependent, non-covalent interaction with a smaller piece of the notch protein composed of a short extracellular region, a single transmembrane-pass, and a small intracellular region.
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-kDa and has 53 amino acid residues and three intramolecular disulfide bonds.

The epidermal growth factor receptor is a transmembrane protein that is a receptor for members of the epidermal growth factor family of extracellular protein ligands.
The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.

Mothers against decapentaplegic homolog 7 or SMAD7 is a protein that in humans is encoded by the SMAD7 gene.

Transforming growth factor alpha (TGF-α) is a protein that in humans is encoded by the TGFA gene. As a member of the epidermal growth factor (EGF) family, TGF-α is a mitogenic polypeptide. The protein becomes activated when binding to receptors capable of protein kinase activity for cellular signaling.
Zalutumumab is a fully human IgG1 monoclonal antibody (mAb) directed towards the epidermal growth factor receptor (EGFR). It is a product developed by Genmab in Utrecht, the Netherlands. Specifically, zalutumumab is designed for the treatment of squamous cell carcinoma of the head and neck (SCCHN), a type of cancer.
The ErbB family of proteins contains four receptor tyrosine kinases, structurally related to the epidermal growth factor receptor (EGFR), its first discovered member. In humans, the family includes Her1, Her2, Her3 (ErbB3), and Her4 (ErbB4). The gene symbol, ErbB, is derived from the name of a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene. Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer's disease, while excessive ErbB signaling is associated with the development of a wide variety of types of solid tumor.

Epiregulin (EPR) is a protein that in humans is encoded by the EREG gene.

Betacellulin is a protein that in humans is encoded by the BTC gene located on chromosome 4 at locus 4q13-q21. Betacellulin was initially identified as a mitogen. Betacellulin, is a part of an Epidermal Growth Factor (EGF) family and functions as a ligand for the epidermal growth factor receptor (EGFR). As the role a EGFR, betacellulin is manifested by different form of muscles and tissues, it also has a great effect of nitrogen that is used for retinal pigment epithelial cells and vascular smooth muscle cells. While many studies attest a role for betacellulin in the differentiation of pancreatic β-cells, the last decade witnessed the association of betacellulin with many additional biological processes, ranging from reproduction to the control of neural stem cells. Betacellulin is a member of the EGF family of growth factors. It is synthesized primarily as a transmembrane precursor, which is then processed to mature molecule by proteolytic events.
Faint little ball (flb) is a Drosophila gene that encodes the Drosophila epidermal growth factor receptor (DER) homolog. The gene is also called torpedo and Ellipse. The gene is located at 3-26 of the Drosophila melanogaster genome. It is named faint little ball because when the gene is mutated the embryo forms a ball of dorsal hypoderm. flb is necessary for several processes to occur during embryonic development, specifically in central nervous system development. It is expressed as quickly as 4 hours after fertilization of the egg. The peak of expression of the flb gene is between 4–8 hours into development. In all processes that are facilitated by flb the same signal transduction pathway is used. Drosophila EGF receptor is involved in the development of embryos as well as larvae/pupae's wings, eyes, legs and ovaries.

Receptor tyrosine-protein kinase erbB-3, also known as HER3, is a membrane bound protein that in humans is encoded by the ERBB3 gene.

Sprouty homolog 2 (Drosophila), also known as SPRY2, is a protein which in humans is encoded by the SPRY2 gene.

EGF-like domain-containing protein 7 is a protein that in humans is encoded by the EGFL7 gene. Intron 7 of EGFL7 hosts the miR-126 microRNA gene.
YWTD repeats are four-stranded beta-propeller repeats found in low-density lipoprotein receptors (LDLR). The six YWTD repeats together fold into a six-bladed beta-propeller. Each blade of the propeller consists of four antiparallel beta-strands; the innermost strand of each blade is labeled 1 and the outermost strand, 4. The sequence repeats are offset with respect to the blades of the propeller, such that any given 40-residue YWTD repeat spans strands 24 of one propeller blade and strand 1 of the subsequent blade. This offset ensures circularization of the propeller because the last strand of the final sequence repeat acts as an innermost strand 1 of the blade that harbors strands 24 from the first sequence repeat. The repeat is found in a variety of proteins that include, vitellogenin receptor from Drosophila melanogaster, low-density lipoprotein (LDL) receptor, preproepidermal growth factor, and nidogen (entactin).

Notch proteins are a family of type-1 transmembrane proteins that form a core component of the Notch signaling pathway, which is highly conserved in metazoans. The Notch extracellular domain mediates interactions with DSL family ligands, allowing it to participate in juxtacrine signaling. The Notch intracellular domain acts as a transcriptional activator when in complex with CSL family transcription factors. Members of this Type 1 transmembrane protein family share several core structures, including an extracellular domain consisting of multiple epidermal growth factor (EGF)-like repeats and an intracellular domain transcriptional activation domain (TAD). Notch family members operate in a variety of different tissues and play a role in a variety of developmental processes by controlling cell fate decisions. Much of what is known about Notch function comes from studies done in Caenorhabditis elegans (C.elegans) and Drosophila melanogaster. Human homologs have also been identified, but details of Notch function and interactions with its ligands are not well known in this context.

A tyrosine kinase inhibitor (TKI) is a pharmaceutical drug that inhibits tyrosine kinases. Tyrosine kinases are enzymes responsible for the activation of many proteins by signal transduction cascades. The proteins are activated by adding a phosphate group to the protein (phosphorylation), a step that TKIs inhibit. TKIs are typically used as anticancer drugs. For example, they have substantially improved outcomes in chronic myelogenous leukemia. They have also been used to treat other diseases, such as idiopathic pulmonary fibrosis.

The rhomboid proteases are a family of enzymes that exist in almost all species. They are proteases: they cut the polypeptide chain of other proteins. This proteolytic cleavage is irreversible in cells, and an important type of cellular regulation. Although proteases are one of the earliest and best studied class of enzyme, rhomboids belong to a much more recently discovered type: the intramembrane proteases. What is unique about intramembrane proteases is that their active sites are buried in the lipid bilayer of cell membranes, and they cleave other transmembrane proteins within their transmembrane domains. About 30% of all proteins have transmembrane domains, and their regulated processing often has major biological consequences. Accordingly, rhomboids regulate many important cellular processes, and may be involved in a wide range of human diseases.

Spitz is a protein in fruit flies which is the major activator of Epidermal Growth Factor Receptor (EGFR).
References
- 1 2 Freeman M, Klämbt C, Goodman CS, Rubin GM (June 1992). "The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye". Cell. 69 (6): 963–75. doi:10.1016/0092-8674(92)90615-j. PMID 1606617. S2CID 17432187.
- ↑ Schweitzer R, Howes R, Smith R, Shilo BZ, Freeman M (August 1995). "Inhibition of Drosophila EGF receptor activation by the secreted protein Argos". Nature. 376 (6542): 699–702. Bibcode:1995Natur.376..699S. doi:10.1038/376699a0. PMID 7651519. S2CID 4343042.
- 1 2 Klein DE, Nappi VM, Reeves GT, Shvartsman SY, Lemmon MA (August 2004). "Argos inhibits epidermal growth factor receptor signalling by ligand sequestration". Nature. 430 (7003): 1040–4. Bibcode:2004Natur.430.1040K. doi:10.1038/nature02840. PMID 15329724. S2CID 4413371.
- ↑ Klein DE, Stayrook SE, Shi F, Narayan K, Lemmon MA (June 2008). "Structural basis for EGFR ligand sequestration by Argos". Nature. 453 (7199): 1271–5. Bibcode:2008Natur.453.1271K. doi:10.1038/nature06978. PMC 2526102 . PMID 18500331.