Related Research Articles

The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. It is the largest site of neural integration in the central nervous system. and plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is the part of the brain responsible for cognition.
Oligodendrocyte progenitor cells (OPCs), also known as oligodendrocyte precursor cells, NG2-glia, O2A cells, or polydendrocytes, are a subtype of glia in the central nervous system named for their essential role as precursors to oligodendrocytes. They are typically identified in the human by co-expression of PDGFRA and CSPG4.
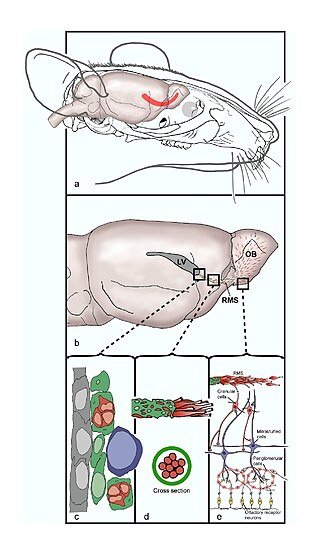
The rostral migratory stream (RMS) is a specialized migratory route found in the brain of some animals along which neuronal precursors that originated in the subventricular zone (SVZ) of the brain migrate to reach the main olfactory bulb (OB). The importance of the RMS lies in its ability to refine and even change an animal's sensitivity to smells, which explains its importance and larger size in the rodent brain as compared to the human brain, as our olfactory sense is not as developed. This pathway has been studied in the rodent, rabbit, and both the squirrel monkey and rhesus monkey. When the neurons reach the OB they differentiate into GABAergic interneurons as they are integrated into either the granule cell layer or periglomerular layer.
Neuroepithelial cells, or neuroectodermal cells, form the wall of the closed neural tube in early embryonic development. The neuroepithelial cells span the thickness of the tube's wall, connecting with the pial surface and with the ventricular or lumenal surface. They are joined at the lumen of the tube by junctional complexes, where they form a pseudostratified layer of epithelium called neuroepithelium.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.

A progenitor cell is a biological cell that can differentiate into a specific cell type. Stem cells and progenitor cells have this ability in common. However, stem cells are less specified than progenitor cells. Progenitor cells can only differentiate into their "target" cell type. The most important difference between stem cells and progenitor cells is that stem cells can replicate indefinitely, whereas progenitor cells can divide only a limited number of times. Controversy about the exact definition remains and the concept is still evolving.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).

Müller glia, or Müller cells, are a type of retinal glial cells, first recognized and described by Heinrich Müller. They are found in the vertebrate retina, where they serve as support cells for the neurons, as all glial cells do. They are the most common type of glial cell found in the retina. While their cell bodies are located in the inner nuclear layer of the retina, they span across the entire retina.

The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.
The Protomap is a primordial molecular map of the functional areas of the mammalian cerebral cortex during early embryonic development, at a stage when neural stem cells are still the dominant cell type. The protomap is a feature of the ventricular zone, which contains the principal cortical progenitor cells, known as radial glial cells. Through a process called 'cortical patterning', the protomap is patterned by a system of signaling centers in the embryo, which provide positional information and cell fate instructions. These early genetic instructions set in motion a development and maturation process that gives rise to the mature functional areas of the cortex, for example the visual, somatosensory, and motor areas. The term protomap was coined by Pasko Rakic. The protomap hypothesis was opposed by the protocortex hypothesis, which proposes that cortical proto-areas initially have the same potential, and that regionalization in large part is controlled by external influences, such as axonal inputs from the thalamus to the cortex. However, a series of papers in the year 2000 and in 2001 provided strong evidence against the protocortex hypothesis, and the protomap hypothesis has been well accepted since then. The protomap hypothesis, together with the related radial unit hypothesis, forms our core understanding of the embryonic development of the cerebral cortex. Once the basic structure is present and cortical neurons have migrated to their final destinations, many other processes contribute to the maturation of functional cortical circuits.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
Endogenous regeneration in the brain is the ability of cells to engage in the repair and regeneration process. While the brain has a limited capacity for regeneration, endogenous neural stem cells, as well as numerous pro-regenerative molecules, can participate in replacing and repairing damaged or diseased neurons and glial cells. Another benefit that can be achieved by using endogenous regeneration could be avoiding an immune response from the host.

A neuronal lineage marker is an endogenous tag that is expressed in different cells along neurogenesis and differentiated cells such as neurons. It allows detection and identification of cells by using different techniques. A neuronal lineage marker can be either DNA, mRNA or RNA expressed in a cell of interest. It can also be a protein tag, as a partial protein, a protein or an epitope that discriminates between different cell types or different states of a common cell. An ideal marker is specific to a given cell type in normal conditions and/or during injury. Cell markers are very valuable tools for examining the function of cells in normal conditions as well as during disease. The discovery of various proteins specific to certain cells led to the production of cell-type-specific antibodies that have been used to identify cells.
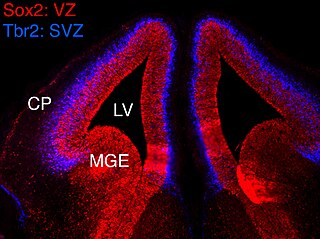
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). In short, it is brain growth in relation to its organization. This occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.

The Radial Unit Hypothesis (RUH) is a conceptual theory of cerebral cortex development, first described by Pasko Rakic. The RUH states that the cerebral cortex develops during embryogenesis as an array of interacting cortical columns, or 'radial units', each of which originates from a transient stem cell layer called the ventricular zone, which contains neural stem cells known as radial glial cells.
Intermediate progenitor cells (IPCs) are a type of progenitor cell in the developing cerebral cortex. They are multipolar cells produced by radial glial cells who have undergone asymmetric division. IPCs can produce neuron cells via neurogenesis and are responsible for ensuring the proper quantity of cortical neurons are produced. In mammals, neural stem cells are the primary progenitors during embryogenesis whereas intermediate progenitor cells are the secondary progenitors.
Arturo Álvarez-Buylla Roces is a professor and endowed chair in neurological surgery, and a researcher in neurobiology at the University of California, San Francisco.
References
- ↑ Pollack, Andrew (2008-05-08). "$271 Million for Research on Stem Cells in California". The New York Times. ISSN 0362-4331 . Retrieved 2019-03-21.
- 1 2 3 "Arnold Kriegstein". alleninstitute.org. Retrieved 2019-03-21.
- 1 2 3 Svendsen, Clive; Ebert, Allison D. (2008). Encyclopedia of Stem Cell Research. SAGE. ISBN 9781412959087.
- ↑ "Californian university tries to cross the great divide in stem cell research". The Irish Times. Retrieved 2019-03-21.
- ↑ Allday, Erin (2011-02-09). "Dolby building is new hub for UCSF stem cell center". SFGate. Retrieved 2019-03-21.
- ↑ Kriegstein, Arnold R.; Dammerman, Ryan S.; Weissman, Tamily A.; Flint, Alexander C.; Noctor, Stephen C. (February 2001). "Neurons derived from radial glial cells establish radial units in neocortex". Nature. 409 (6821): 714–720. Bibcode:2001Natur.409..714N. doi:10.1038/35055553. ISSN 1476-4687. PMID 11217860. S2CID 3041502.
- ↑ Kriegstein, Arnold R.; Lidija Ivic; Martínez-Cerdeño, Verónica; Noctor, Stephen C. (February 2004). "Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases". Nature Neuroscience. 7 (2): 136–144. doi:10.1038/nn1172. ISSN 1546-1726. PMID 14703572. S2CID 15946842.
- ↑ Kriegstein, Arnold R.; Philip R. L. Parker; Lui, Jan H.; Hansen, David V. (March 2010). "Neurogenic radial glia in the outer subventricular zone of human neocortex". Nature. 464 (7288): 554–561. Bibcode:2010Natur.464..554H. doi:10.1038/nature08845. ISSN 1476-4687. PMID 20154730. S2CID 4412132.
- ↑ Lui, Jan H. (2011). "Development and Evolution of the Human Neocortex". Cell. 146 (1): 18–36. doi:10.1016/j.cell.2011.06.030. PMC 3610574 . PMID 21729779.
- ↑ "Solomon A. Berson Medical Alumni Achievement". NYU Langone Health. Retrieved 2019-03-21.
- ↑ Svendsen, Clive; Ebert, Allison D. (2008). Encyclopedia of Stem Cell Research. SAGE. ISBN 9781412959087.
- ↑ "NINDS Research Program Award (R35) Recipients FY 2017 | National Institute of Neurological Disorders and Stroke". www.ninds.nih.gov. Retrieved 2019-03-21.