
In organic chemistry, a ketone is a functional group with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
An isocyanide is an organic compound with the functional group –N+≡C−. It is the isomer of the related nitrile (–C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.
In organic chemistry, the Knoevenagel condensation reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way.

1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2)2CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonia in the presence of potassium cyanide. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used commercially for the production of racemic methionine from methional.
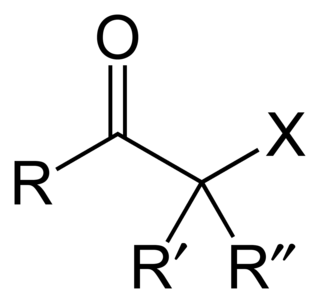
In organic chemistry, an α-haloketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-haloketones are alkylating agents. Prominent α-haloketones include phenacyl bromide and chloroacetone.
The Meerwein–Ponndorf–Verley (MPV) reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminium alkoxide catalysis in the presence of a sacrificial alcohol. The advantages of the MPV reduction lie in its high chemoselectivity, and its use of a cheap environmentally friendly metal catalyst.
The Andrussow process is an industrial process for the production of hydrogen cyanide from methane and ammonia in the presence of oxygen and a platinum catalyst.
The Chichibabin pyridine synthesis is a method for synthesizing pyridine rings. The reaction involves the condensation reaction of aldehydes, ketones, α,β-Unsaturated carbonyl compounds, or any combination of the above, with ammonia. It was reported by Aleksei Chichibabin in 1924. Methyl-substituted pyridines, which show widespread uses among multiple fields of applied chemistry, are prepared by this methodology.
The BMA process or Degussa process is a chemical process developed by the German chemical company Degussa for the production of hydrogen cyanide from methane and ammonia in presence of a platinum catalyst. Hydrogen cyanide is used in the chemical industry for the production of intermediate chemicals like acrylonitrile, methyl methacrylate, and adiponitrile.

Friedrich Asinger was an Austrian chemist and professor for Technical Chemistry. He is well known for his development of a multi-component reaction, the Asinger reaction for the synthesis of 3-thiazolines.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Heribert Offermanns is a German chemist and former member of the board of the Degussa AG.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.

Tris(dimethylamino)methane (TDAM) is the simplest representative of the tris(dialkylamino)methanes of the general formula (R2N)3CH in which three of the four of methane's hydrogen atoms are replaced by dimethylamino groups (−N(CH3)2). Tris(dimethylamino)methane can be regarded as both an amine and an orthoamide.

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ-R. Such compounds include enones and enals. In these compounds the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.

Jieping Zhu is an organic chemist specializing in natural product total synthesis and organometallics. He is a professor of chemistry at EPFL and the head of the Laboratory of Synthesis and Natural Products.













