
In organic chemistry a diene is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alkene units, with the standard prefix di of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition.

Organic chemistry is a branch of chemistry that studies the structure, properties and reactions of organic compounds, which contain carbon in covalent bonding. Study of structure determines their chemical composition and formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and tin, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula HOOC-CH-(NH2)-CH2-SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.

In chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. Chemical compounds containing such rings are also referred to as furans.

Imidazole is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).

Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
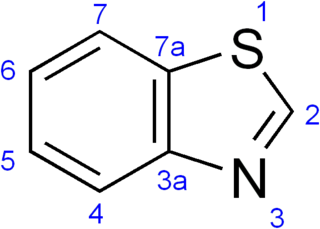
Benzothiazole is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.
Thiazolidine is a heterocyclic organic compound with the formula (CH2)3(NH)S. It is a 5-membered saturated ring with a thioether group and an amine group in the 1 and 3 positions. It is a sulfur analog of oxazolidine. Thiazolidine is a colorless liquid.

Bredt's rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. It primarily relates to bridgeheads with carbon-carbon and carbon-nitrogen double bonds.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsine and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.

Oxazoline is a five-membered heterocyclic chemical compound containing one atom each of oxygen and nitrogen. It was likely first synthesized in 1884 but it was not until 5 years later that Siegmund Gabriel correctly assigned the structure. It was named in-line with the Hantzsch–Widman nomenclature and is part of a family of heterocyclic compounds, where it exists between oxazole and oxazolidine in terms of saturation.
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions.

Thiopeptides are a class of peptide antibiotics produced by bacteria. They have antibiotic activity against Gram-positive bacteria, but little or no activity against Gram-negative bacteria. Many of the members of this class show activity against methicillin-resistant Staphylococcus aureus (MRSA) and are therefore subjects of research interest.
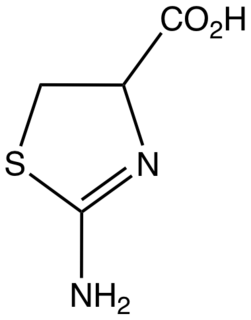
2-Aminothiazoline-4-carboxylic acid (ACTA) is the organosulfur compound and a heterocycle with the formula HO2CCHCH2SCNH2N. This derivative of thiazoline is an intermediate in the industrial synthesis of L-cysteine, an amino acid. ACTA exists in equilibrium with its tautomer 2-iminothiazolidine-4-carboxylic acid.















