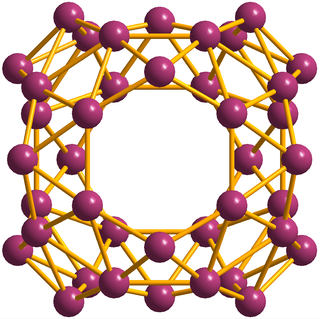
Azafullerenes are a class of heterofullerenes in which the element substituting for carbon is nitrogen. They can be in the form of a hollow sphere, ellipsoid, tube, and many other shapes. Spherical azafullerenes resemble the balls used in football (soccer). They are also a member of the carbon nitride class of materials that include beta carbon nitride (β-C3N4), predicted to be harder than diamond. Besides the pioneering work of a couple of academic groups, this class of compounds has so far garnered little attention from the broader fullerene research community. Many properties and structures are yet to be discovered for the highly-nitrogen substituted subset of molecules.
Heterofullerenes are classes of fullerenes, at least one carbon atom is replaced by another element. Based on spectroscopy, substitutions have been reported with boron (borafullerenes), nitrogen (azafullerenes), oxygen, arsenic, germanium, phosphorus, silicon, iron, copper, nickel, rhodium and iridium. Reports on isolated heterofullerenes are limited to those based on nitrogen and oxygen.

Carbon is a chemical element with symbol C and atomic number 6. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.

Nitrogen is a chemical element with symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790, when it was found that nitrogen was present in nitric acid and nitrates. Antoine Lavoisier suggested instead the name azote, from the Greek ἀζωτικός "no life", as it is an asphyxiant gas; this name is instead used in many languages, such as French, Russian, Romanian and Turkish, and appears in the English names of some nitrogen compounds such as hydrazine, azides and azo compounds.
The first fullerene molecule to be discovered, and the family's namesake, buckminsterfullerene (C60), was prepared in 1985 by Richard Smalley, Robert Curl, James Heath, Sean O'Brien, and Harold Kroto at Rice University. [1] A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, tube, and many other shapes. Spherical fullerenes are also called buckyballs, and they resemble the balls used in football (soccer). Fullerenes are similar in structure to graphite, which is composed of stacked graphene sheets of linked hexagonal rings; but they may also contain pentagonal (or sometimes heptagonal) rings.

A fullerene is an allotrope of carbon in the form of a hollow sphere, ellipsoid, tube, and many other shapes and sizes. Spherical fullerenes, also referred to as Buckminsterfullerenes or buckyballs, resemble the balls used in association football. Cylindrical fullerenes are also called carbon nanotubes (buckytubes). Fullerenes are similar in structure to graphite, which is composed of stacked graphene sheets of linked hexagonal rings. Unless they are cylindrical, they must also contain pentagonal rings.

Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) that resembles a soccer ball (football), made of twenty hexagons and twelve pentagons, with a carbon atom at each vertex of each polygon and a bond along each polygon edge.

Richard Errett Smalley was the Gene and Norman Hackerman Professor of Chemistry and a Professor of Physics and Astronomy at Rice University, in Houston, Texas. In 1996, along with Robert Curl, also a professor of chemistry at Rice, and Harold Kroto, a professor at the University of Sussex, he was awarded the Nobel Prize in Chemistry for the discovery of a new form of carbon, buckminsterfullerene, also known as buckyballs. He was an advocate of nanotechnology and its applications.
Azafullerenes were first discovered in 1993 and reported in the California State Science Fair. [2] The derivatives were formed in the gap between two graphite rods connected to an electric power supply. A small air leak led to contamination of the inert atmosphere and the subsequent reaction. The materials can also be formed by chemical reactions on fullerene or laser ablation of graphitic materials.
The California State Science Fair is a science fair held annually at the California Science Center in Los Angeles.
Subsequent work revealed a wide range of carbon nitride structures. [3] [4] [5] Examples include (C59N)2 (biazafullerenyl),C58N2 (diaza[60]fullerene), C57N3 (triaza[60]fullerene) and C48N12. The nitrogen atoms substitute for carbon atoms on the cage-like molecules. Much of the work has been theoretical in nature. The C48N12 molecule was calculated to be an insulator, with the eight all-carbon rings forming regions of extended electron delocalization. [6]
Carbon nitrides are compounds of carbon and nitrogen.