Related Research Articles

A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene, which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family.

Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) that resembles a soccer ball, made of twenty hexagons and twelve pentagons. Each carbon atom has three bonds. It is a black solid that dissolves in hydrocarbon solvents to produce a violet solution. The compound has received intense study, although few real world applications have been found.
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.

Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex was synthesized in 1985 and called La@C60. The @ (at sign) in the name reflects the notion of a small molecule trapped inside a shell. Two types of endohedral complexes exist: endohedral metallofullerenes and non-metal doped fullerenes.

The Prato reaction is a particular example of the well-known 1,3-dipolar cycloaddition of azomethine ylides to olefins. In fullerene chemistry this reaction refers to the functionalization of fullerenes and nanotubes. The amino acid sarcosine reacts with paraformaldehyde when heated at reflux in toluene to an ylide which reacts with a double bond in a 6,6 ring position in a fullerene via a 1,3-dipolar cycloaddition to yield a N-methylpyrrolidine derivative or pyrrolidinofullerene or pyrrolidino[[3,4:1,2]] [60]fullerene in 82% yield based on C60 conversion.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.

A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces, dipole-dipole interactions, quadrupole interactions, π-π interactions, hydrogen bonding, halogen bonding, London dispersion forces, and in some molecular solids, coulombic interactions. Van der Waals, dipole interactions, quadrupole interactions, π-π interactions, hydrogen bonding, and halogen bonding are typically much weaker than the forces holding together other solids: metallic, ionic, and network solids. Intermolecular interactions, typically do not involve delocalized electrons, unlike metallic and certain covalent bonds. Exceptions are charge-transfer complexes such as the tetrathiafulvane-tetracyanoquinodimethane (TTF-TCNQ), a radical ion salt. These differences in the strength of force and electronic characteristics from other types of solids give rise to the unique mechanical, electronic, and thermal properties of molecular solids.
This page deals with the electron affinity as a property of isolated atoms or molecules. Solid state electron affinities are not listed here.

PCBM is the common abbreviation for the fullerene derivative [6,6]-phenyl-C61-butyric acid methyl ester. It is being investigated in organic solar cells.
Organic photovoltaic devices (OPVs) are fabricated from thin films of organic semiconductors, such as polymers and small-molecule compounds, and are typically on the order of 100 nm thick. Because polymer based OPVs can be made using a coating process such as spin coating or inkjet printing, they are an attractive option for inexpensively covering large areas as well as flexible plastic surfaces. A promising low cost alternative to conventional solar cells made of crystalline silicon, there is a large amount of research being dedicated throughout industry and academia towards developing OPVs and increasing their power conversion efficiency.
Mitsutaka Fujita was a Japanese physicist. He proposed the edge state that is unique to graphene zigzag edges. Also, he theoretically pointed out the importance and peculiarity of nanoscale and edge shape effects in nanographene. The theoretical concept of graphene nanoribbons was introduced by him and his research group to study the nanoscale effect of graphene. He was an associate professor at Tsukuba University, and died of a subarachnoid hemorrhage on March 18, 1998. His posthumous name is Rikakuin-Shinju-Houkou-Koji (理覚院深珠放光居士) in Japanese.
Carbon nitrides are compounds of carbon and nitrogen.

Rodney S. "Rod" Ruoff is an American physical chemist and nanoscience researcher. He is one of the world experts on carbon materials including carbon nanostructures such as fullerenes, nanotubes, graphene, diamond, and has had pioneering discoveries on such materials and others. Ruoff received his B.S. in Chemistry from the University of Texas at Austin (1981) and his Ph.D. in Chemical Physics at the University of Illinois-Urbana (1988). After a Fulbright Fellowship at the MPI fuer Stroemungsforschung in Goettingen, Germany (1989) and postdoctoral work at the IBM T. J. Watson Research Center (1990–91), Ruoff became a staff scientist in the Molecular Physics Laboratory at SRI International (1991-1996). He is currently UNIST Distinguished Professor at the Ulsan National Institute of Science and Technology (UNIST), and the director of the Center for Multidimensional Carbon Materials, an Institute for Basic Science Center located at UNIST.
The Buchner ring expansion is a two-step organic C-C bond forming reaction used to access 7-membered rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanates an aromatic ring. The ring expansion occurs in the second step, with an electrocyclic reaction opening the cyclopropane ring to form the 7-membered ring.

A transition metal fullerene complex is a coordination complex wherein fullerene serves as a ligand. Fullerenes are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene, C60.
Topological inhibitors are rigid three-dimensional molecules of inorganic, organic and hybrid compounds that form multicentered supramolecular interactions in vacant cavities of protein macromolecules and their complexes . Extensive surface and very diverse geometry make cage compounds with an encapsulated metal ion (clathrochelates) suitable for targeting both the active and allosteric sites of enzymes as well as the interfaces of their macromolecular complexes. An efficient structure- and concentration-dependent transcription inhibition in a model in vitro systems based on RNA and DNA polymerases by the iron(II) mono- and bis-clathrochelates at their submicro- and nanomolar concentrations, respectively, is observed in. Molecular docking and preincubation experiments suggested that these cage compounds form supramolecular assemblies with protein residues as well as with DNA and RNA. Thus, they are prospective precursors for the design of antiviral and anticancer drug candidates.
Azafullerenes are a class of heterofullerenes in which the element substituting for carbon is nitrogen. They can be in the form of a hollow sphere, ellipsoid, tube, and many other shapes. Spherical azafullerenes resemble the balls used in football (soccer). They are also a member of the carbon nitride class of materials that include beta carbon nitride (β-C3N4), predicted to be harder than diamond. Besides the pioneering work of a couple of academic groups, this class of compounds has so far garnered little attention from the broader fullerene research community. Many properties and structures are yet to be discovered for the highly-nitrogen substituted subset of molecules.
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.

Carbon peapod is a hybrid nanomaterial consisting of spheroidal fullerenes encapsulated within a carbon nanotube. It is named due to their resemblance to the seedpod of the pea plant. Since the properties of carbon peapods differ from those of nanotubes and fullerenes, the carbon peapod can be recognized as a new type of a self-assembled graphitic structure. Possible applications of nano-peapods include nanoscale lasers, single electron transistors, spin-qubit arrays for quantum computing, nanopipettes, and data storage devices thanks to the memory effects and superconductivity of nano-peapods.
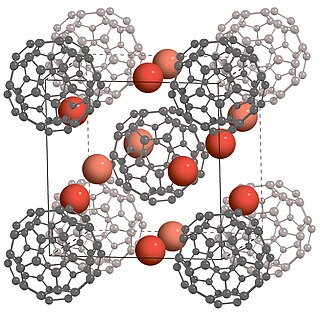
Fullerides are chemical compounds containing fullerene anions. Common fullerides are derivatives of the most common fullerenes, i.e. C60 and C70. The scope of the area is large because multiple charges are possible, i.e., [C60]n− (n = 1, 2...6), and all fullerenes can be converted to fullerides. The suffix "-ide" implies their negatively charged nature.
References
- ↑ Vostrowsky, O.; Hirsch, A. (2006). "Heterofullerenes". Chemical Reviews. 106 (12): 5191–5207. doi:10.1021/cr050561e. PMID 17165685.
- ↑ Hummelen, Jan C.; Bellavia-Lund, Cheryl; Wudl, Fred (1999). "Heterofullerenes. Fullerenes and Related Structures". Topics in Current Chemistry. 199: 93–134. doi:10.1007/3-540-68117-5_3.
- ↑ Chai, Y.; Guo, T.; Jin, C.; Haufler, R. E.; Chibante, L. P. F.; Fure, J.; Wang, L.; Alford, J. M.; Smalley, R. E. (1991). "Fullerenes with metals inside". The Journal of Physical Chemistry. 95 (20): 7564. doi:10.1021/j100173a002.
- ↑ Muhr, H. -J.; Nesper, R.; Schnyder, B.; Kötz, R. (1996). "The boron heterofullerenes C59B and C69B: Generation, extraction, mass spectrometric and XPS characterization". Chemical Physics Letters. 249 (5–6): 399. Bibcode:1996CPL...249..399M. doi:10.1016/0009-2614(95)01451-9.
- ↑ Averdung, J.; Luftmann, H.; Schlachter, I.; Mattay, J. (1995). "Aza-dihydro[60]fullerene in the gas phase. A mass-spectrometric and quantumchemical study". Tetrahedron. 51 (25): 6977. doi:10.1016/0040-4020(95)00361-B.
- ↑ Lamparth, I.; Nuber, B.; Schick, G.; Skiebe, A.; Grösser, T.; Hirsch, A. (1995). "C59N+ and C69N+: Isoelectronic Heteroanalogues of C60 and C70". Angewandte Chemie International Edition in English. 34 (20): 2257. doi:10.1002/anie.199522571.
- ↑ Christian, J. F.; Wan, Z.; Anderson, S. L. (1992). "O++C60•C60O+ production and decomposition, charge transfer, and formation of C59O+. Dopeyball or [CO@C58]+". Chemical Physics Letters. 199 (3–4): 373. Bibcode:1992CPL...199..373C. doi:10.1016/0009-2614(92)80134-W.
- ↑ Ohtsuki, T.; Ohno, K.; Shiga, K.; Kawazoe, Y.; Maruyama, Y.; Masumoto, K. (1999). "Formation of As- and Ge-doped heterofullerenes". Physical Review B. 60 (3): 1531. Bibcode:1999PhRvB..60.1531O. doi:10.1103/PhysRevB.60.1531.
- ↑ Möschel, C.; Jansen, M. (1999). "Darstellung stabiler Phosphor-Heterofullerene im Hochfrequenzofen". Z. Anorg. Allg. Chem. 625 (2): 175–177. doi:10.1002/(SICI)1521-3749(199902)625:2<175::AID-ZAAC175>3.0.CO;2-2.
- ↑ Pellarin, M.; Ray, C.; Lermé, J.; Vialle, J. L.; Broyer, M.; Blase, X.; Kéghélian, P.; Mélinon, P.; Perez, A. (1999). "Photolysis experiments on SiC mixed clusters: From silicon carbide clusters to silicon-doped fullerenes". The Journal of Chemical Physics. 110 (14): 6927–6938. Bibcode:1999JChPh.110.6927P. doi:10.1063/1.478598.
- 1 2 3 Billas, I.M.L.; Branz, W.; Malinowski, N.; Tast, F.; Heinebrodt, M.; Martin, T.P.; Massobrio, C.; Boero, M.; Parrinello, M. (1999). "Experimental and computational studies of heterofullerenes". Nanostructured Materials. 12 (5–8): 1071–1076. doi:10.1016/S0965-9773(99)00301-3.
- ↑ Branz, W.; Billas, I. M. L.; Malinowski, N.; Tast, F.; Heinebrodt, M.; Martin, T. P. (1998). "Cage substitution in metal–fullerene clusters". The Journal of Chemical Physics. 109 (9): 3425. Bibcode:1998JChPh.109.3425B. doi:10.1063/1.477410.
- ↑ Harris, D.J. (1993) "Discovery of Nitroballs: Research in Fullerene Chemistry" http://www.usc.edu/CSSF/History/1993/CatWin_S05.html Archived 2015-11-29 at the Wayback Machine
- ↑ Hummelen, J. C.; Knight, B.; Pavlovich, J.; Gonzalez, R.; Wudl, F. (1995). "Isolation of the Heterofullerene C59N as Its Dimer (C59N)2" (PDF). Science. 269 (5230): 1554–1556. Bibcode:1995Sci...269.1554H. doi:10.1126/science.269.5230.1554. hdl: 11370/ffe5ba8c-5336-4aed-9c78-0a26f31d459d . PMID 17789446.
- ↑ Keshavarz-K, M.; González, R.; Hicks, R. G.; Srdanov, G.; Srdanov, V. I.; Collins, T. G.; Hummelen, J. C.; Bellavia-Lund, C.; Pavlovich, J.; Wudl, F.; Holczer, K. (1996). "Synthesis of hydroazafullerene C59HN, the parent hydroheterofullerene". Nature. 383 (6596): 147. Bibcode:1996Natur.383..147K. doi:10.1038/383147a0.
- ↑ Nuber, B.; Hirsch, A. (1996). "A new route to nitrogen heterofullerenes and the first synthesis of (C69N)2". Chemical Communications (12): 1421. doi:10.1039/CC9960001421.
- ↑ Zhang, G.; Huang, S.; Xiao, Z.; Chen, Q.; Gan, L.; Wang, Z. (2008). "Preparation of Azafullerene Derivatives from Fullerene-Mixed Peroxides and Single Crystal X-ray Structures of Azafulleroid and Azafullerene". Journal of the American Chemical Society. 130 (38): 12614–12615. doi:10.1021/ja805072h. PMID 18759401.
- ↑ Xin, N.; Huang, H.; Zhang, J.; Dai, Z.; Gan, L. (2012). "Fullerene Doping: Preparation of Azafullerene C59NH and Oxafulleroids C59O3 and C60O4". Angewandte Chemie International Edition. 51 (25): 6163–6166. doi:10.1002/anie.201202777. PMID 22573566.