Related Research Articles

Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.

In chemistry, cyanide is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely divided iron metal as a catalyst:

The Miller–Urey experiment, or Miller experiment, was an experiment in chemical synthesis carried out in 1952 that simulated the conditions thought at the time to be present in the atmosphere of the early, prebiotic Earth. It is seen as one of the first successful experiments demonstrating the synthesis of organic compounds from inorganic constituents in an origin of life scenario. The experiment used methane (CH4), ammonia (NH3), hydrogen (H2), in ratio 2:2:1, and water (H2O). Applying an electric arc (simulating lightning) resulted in the production of amino acids.
Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N. It is a highly toxic and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
Coal gas is a flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. It is produced when coal is heated strongly in the absence of air. Town gas is a more general term referring to manufactured gaseous fuels produced for sale to consumers and municipalities.
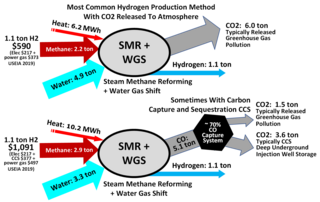
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:

The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.
A methane reformer is a device based on steam reforming, autothermal reforming or partial oxidation and is a type of chemical synthesis which can produce pure hydrogen gas from methane using a catalyst. There are multiple types of reformers in development but the most common in industry are autothermal reforming (ATR) and steam methane reforming (SMR). Most methods work by exposing methane to a catalyst at high temperature and pressure.

Leonid Andrussow was a German chemical engineer. He developed the process for the production of hydrogen cyanide based on the oxidation of ammonia and methane, which is named after him Andrussow oxidation.

The Andrussow process is the dominant industrial process for the production of hydrogen cyanide. It involves the reaction of methane, ammonia, and oxygen. The process is catalyzed by a platinum-rhodium alloy.
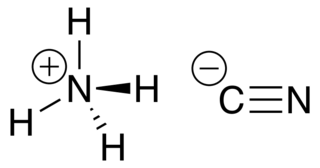
Ammonium cyanide is an unstable inorganic compound with the formula NH4CN.
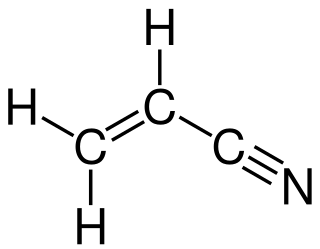
In organic chemistry, ammoxidation is a process for the production of nitriles using ammonia and oxygen. It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:

Endothermic gas is a gas that inhibits or reverses oxidation on the surfaces it is in contact with. This gas is the product of incomplete combustion in a controlled environment. An example mixture is hydrogen gas (H2), nitrogen gas (N2), and carbon monoxide (CO). The hydrogen and carbon monoxide are reducing agents, so they work together to shield surfaces from oxidation.

Nitrilotriacetonitrile (NTAN) is a precursor for nitrilotriacetic acid, for tris(2-aminoethyl)amine and for the epoxy resin crosslinker aminoethylpiperazine.
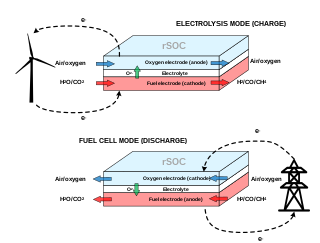
A reversible solid oxide cell (rSOC) is a solid-state electrochemical device that is operated alternatively as a solid oxide fuel cell (SOFC) and a solid oxide electrolysis cell (SOEC). Similarly to SOFCs, rSOCs are made of a dense electrolyte sandwiched between two porous electrodes. Their operating temperature ranges from 600°C to 900°C, hence they benefit from enhanced kinetics of the reactions and increased efficiency with respect to low-temperature electrochemical technologies.
Magnesium cyanide is a chemical compound with the formula Mg(CN)2. It is a toxic white solid. Unlike calcium isocyanide, the cyanide ligands prefer to coordinate at carbon, with a 0.3‑kcal/mol isomerization barrier. When this salt is heated to 500 °C, it decomposes to magnesium nitride.
References
- ↑ patent literature Archived 2012-09-06 at archive.today
- ↑ F. Endter (1958). "Die technische Synthese von Cyanwasserstoff aus Methan und Ammoniak ohne Zusatz von Sauerstoff". Chemie Ingenieur Technik. 30 (5): 281–376. doi:10.1002/cite.330300506.