An intron is any nucleotide sequence within a gene that is removed by RNA splicing during maturation of the final RNA product. In other words, introns are non-coding regions of an RNA transcript, or the DNA encoding it, that are eliminated by splicing before translation. The word intron is derived from the term intragenic region, i.e. a region inside a gene. The term intron refers to both the DNA sequence within a gene and the corresponding sequence in RNA transcripts. Sequences that are joined together in the final mature RNA after RNA splicing are exons.

RNA splicing is a process in molecular biology where a newly-made precursor messenger RNA (pre-mRNA) transcript is transformed into a mature messenger RNA (mRNA). It works by removing introns and so joining together exons. For nuclear-encoded genes, splicing occurs in the nucleus either during or immediately after transcription. For those eukaryotic genes that contain introns, splicing is usually needed to create an mRNA molecule that can be translated into protein. For many eukaryotic introns, splicing occurs in a series of reactions which are catalyzed by the spliceosome, a complex of small nuclear ribonucleoproteins (snRNPs). There exist self-splicing introns, that is, ribozymes that can catalyze their own excision from their parent RNA molecule.

Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be included within or excluded from the final, processed messenger RNA (mRNA) produced from that gene. This means the exons are joined in different combinations, leading to different (alternative) mRNA strands. Consequently, the proteins translated from alternatively spliced mRNAs will contain differences in their amino acid sequence and, often, in their biological functions. Notably, alternative splicing allows the human genome to direct the synthesis of many more proteins than would be expected from its 20,000 protein-coding genes.

Ribozymes are RNA molecules that have the ability to catalyze specific biochemical reactions, including RNA splicing in gene expression, similar to the action of protein enzymes. The 1982 discovery of ribozymes demonstrated that RNA can be both genetic material and a biological catalyst, and contributed to the RNA world hypothesis, which suggests that RNA may have been important in the evolution of prebiotic self-replicating systems. The most common activities of natural or in vitro-evolved ribozymes are the cleavage or ligation of RNA and DNA and peptide bond formation. Within the ribosome, ribozymes function as part of the large subunit ribosomal RNA to link amino acids during protein synthesis. They also participate in a variety of RNA processing reactions, including RNA splicing, viral replication, and transfer RNA biosynthesis. Examples of ribozymes include the hammerhead ribozyme, the VS ribozyme, Leadzyme and the hairpin ribozyme.
The 5′ untranslated region is the region of a messenger RNA (mRNA) that is directly upstream from the initiation codon. This region is important for the regulation of translation of a transcript by differing mechanisms in viruses, prokaryotes and eukaryotes. While called untranslated, the 5′ UTR or a portion of it is sometimes translated into a protein product. This product can then regulate the translation of the main coding sequence of the mRNA. In many organisms, however, the 5′ UTR is completely untranslated, instead forming complex secondary structure to regulate translation.
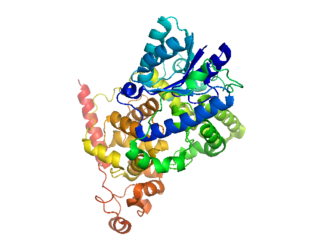
Cryptochromes are a class of flavoproteins found in plants and animals that are sensitive to blue light. They are involved in the circadian rhythms and the sensing of magnetic fields in a number of species. The name cryptochrome was proposed as a portmanteau combining the cryptic nature of the photoreceptor, and the cryptogamic organisms on which many blue-light studies were carried out.

U1 spliceosomal RNA is the small nuclear RNA (snRNA) component of U1 snRNP, an RNA-protein complex that combines with other snRNPs, unmodified pre-mRNA, and various other proteins to assemble a spliceosome, a large RNA-protein molecular complex upon which splicing of pre-mRNA occurs. Splicing, or the removal of introns, is a major aspect of post-transcriptional modification, and takes place only in the nucleus of eukaryotes.

U2 spliceosomal snRNAs are a species of small nuclear RNA (snRNA) molecules found in the major spliceosomal (Sm) machinery of virtually all eukaryotic organisms. In vivo, U2 snRNA along with its associated polypeptides assemble to produce the U2 small nuclear ribonucleoprotein (snRNP), an essential component of the major spliceosomal complex. The major spliceosomal-splicing pathway is occasionally referred to as U2 dependent, based on a class of Sm intron—found in mRNA primary transcripts—that are recognized exclusively by the U2 snRNP during early stages of spliceosomal assembly. In addition to U2 dependent intron recognition, U2 snRNA has been theorized to serve a catalytic role in the chemistry of pre-RNA splicing as well. Similar to ribosomal RNAs (rRNAs), Sm snRNAs must mediate both RNA:RNA and RNA:protein contacts and hence have evolved specialized, highly conserved, primary and secondary structural elements to facilitate these types of interactions.

X-box binding protein 1, also known as XBP1, is a protein which in humans is encoded by the XBP1 gene. The XBP1 gene is located on chromosome 22 while a closely related pseudogene has been identified and localized to chromosome 5. The XBP1 protein is a transcription factor that regulates the expression of genes important to the proper functioning of the immune system and in the cellular stress response.
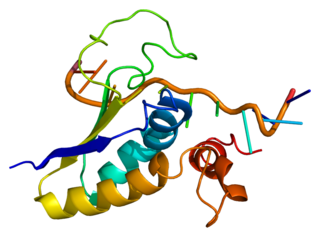
Splicing factor 1 also known as zinc finger protein 162 (ZFM162) is a protein that in humans is encoded by the SF1 gene.
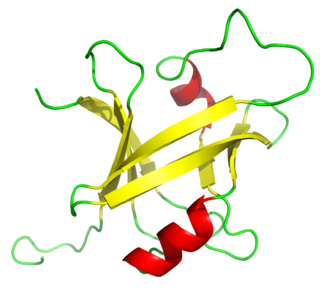
The B3 DNA binding domain (DBD) is a highly conserved domain found exclusively in transcription factors combined with other domains. It consists of 100-120 residues, includes seven beta strands and two alpha helices that form a DNA-binding pseudobarrel protein fold ; it interacts with the major groove of DNA.

The bZIP intron RNA motif is an RNA structure guiding splicing of a non-canonical intron from bZIP-containing genes called HAC1 in yeast, XBP1 in Metazoa, Hxl1 or Cib1 in Basidiomycota and bZIP60 in plants. Splicing is performed independently of the spliceosome by Ire1, a kinase with endoribonuclease activity. Exons are joined by a tRNA ligase. Recognition of the intron splice sites is mediated by a base-paired secondary structure of the mRNA that forms at the exon/intron boundaries. Splicing of the bZIP intron is a key regulatory step in the unfolded protein response (UPR). The Ire-mediated unconventional splicing was first described for HAC1 in S. cerevisiae.
Periannan Senapathy is a molecular biologist, geneticist, author and entrepreneur. He is the founder, president and chief scientific officer at Genome International Corporation, a biotechnology, bioinformatics, and information technology firm based in Madison, Wisconsin, which develops computational genomics applications of next-generation DNA sequencing (NGS) and clinical decision support systems for analyzing patient genome data that aids in diagnosis and treatment of diseases.
Epstein–Barr virus stable intronic-sequence RNAs (ebv-sisRNAs) are a class of non-coding RNAs generated by repeat introns in the Epstein–Barr virus. After EBERs 1 and 2, ebv-sisRNA-1 is the third most abundant EBV RNA generated during a highly oncogenic form of virus latency. Conservation of ebv-sisRNA sequence and secondary structure between EBV and other herpesviruses suggest shared functions in latent infection.
The split gene theory is a theory of the origin of introns, long non-coding sequences in eukaryotic genes between the exons. The theory holds that the randomness of primordial DNA sequences would only permit small (< 600bp) open reading frames (ORF), and that important intron structures and regulatory sequences are derived from stop codons. In this introns-first framework, the spliceosomal machinery and the nucleus evolved due to the necessity to join these ORFs into larger proteins, and that intronless bacterial genes are less ancestral than the split eukaryotic genes. The theory originated with Periannan Senapathy.

The bZIP intron animal is an unconventional bZIP intron in animals located in the mRNA of Xbp1 orthologs. The RNA structure consists of two hairpins of similar length with loop regions defining the splice sites. Intron is usually 23 or 26 nt long and it is excised by endoribonuclease Ire1 encoded by ERN1 gene in response to ER stress. The splicing mechanism in this group was first reported in human.

The bZIP intron ascomycota is an unconventional bZIP intron found in some of the Ascomycota fungi, mainly in filamentous fungi from Pezizomycotina subphylum. The structure consists of two hairpins: a longer on at the 5′ and a shorter one at the 3’. Loop regions of the hairpins define the position of splice sites recognised by endoribonuclease Ire1 in response to ER stress. The unconventional splicing in this group results in excising introns of typical length 20 or 23 nt and it was first described in Trichoderma reesei and Aspergillus nidulans hacA mRNAs.

The bZIP intron basidiomycota is an unconventional bZIP intron found mainly in the Basidiomycota and some Mucoromycotina fungi. The consensus RNA structure is formed by three hairpins - two well conserved at the 5’ and 3’ ends and a variable one in between them. The loop regions of 5’ and 3’ hairpins define the splice sites recognised by Ire1, which performs the unconventional splicing in response to ER stress. In Basidiomycota, splicing results in excised introns from 20 to 101 nt in length and it was first described in Cryptococcus neoformans.

The bZIP intron candida is an unconventional bZIP intron located in the HAC1 mRNA in a subgroup of fungi from Saccharomycetales order. So far all species with this type of structure belong to Metschnikowiaceae or Debaryomycetaceae families. However, some of the best known representatives of Debaryomycetaceae - Candida albicans and its closest relatives - contain the shorter RNA structure instead. The consensus structure consists of two well conserved hairpins with loop regions defining the unconventional splice sites. The hairpins are separated by a long insertion with conserved motifs and a predicted secondary structure. Splicing performed by Ire1 results in excision of a very long intron that was first described in Candida parapsilosis.

The bZIP intron saccharomycetales is an unconventional bZIP intron located in the HAC1 mRNA in most budding yeast belonging to Saccharomycetales order. The structure consists of two hairpins with their loop regions defining 5’ and 3’ splice sites and a long, poorly conserved sequence separating them. In some species this poorly conserved region can pair with the 5’ UTR of the HAC1 mRNA forming a pseudoknot, which stalls the translation. The unconventional splicing is performed by an endoribonuclease Ire1 in response to ER stress and it was first shown in Saccharomyces cerevisiae.















