Elias James Corey is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.
The aldol reaction is a reaction that combines two carbonyl compounds to form a new β-hydroxy carbonyl compound.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."

The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which a prochiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine reagent which mediates the enantioselective reduction of ketones was previously developed by the laboratory of Itsuno and thus this transformation may more properly be called the Itsuno-Corey oxazaborolidine reduction.

The CBS catalyst or Corey–Bakshi–Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and (3+2) cycloadditions. Proline, a naturally occurring chiral compound, is readily and cheaply available. It transfers its stereocenter to the catalyst which in turn is able to drive an organic reaction selectively to one of two possible enantiomers. This selectivity is due to steric strain in the transition state that develops for one enantiomer but not for the other.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

Lisofylline (LSF) is a synthetic small molecule with novel anti-inflammatory properties. LSF can effectively prevent type 1 diabetes in preclinical models and improves the function and viability of isolated or transplanted pancreatic islets. It is a metabolite of pentoxifylline.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.

A boronic acid is an organic compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes.
Prolinol is a chiral amino-alcohol that is used as a chiral building block in organic synthesis. It exists as two enantiomers: the D and L forms.

Diisopinocampheylborane is an organoborane that is useful for asymmetric synthesis. This colourless solid is the precursor to a range of related reagents. The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes. The reagent is mainly used for the synthesis of chiral secondary alcohols.

Borane dimethylsulfide (BMS) is a chemical compound with the chemical formula BH3·S(CH3)2. It is an adduct between borane molecule and dimethyl sulfide molecule. It is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, BH3·THF requires sodium borohydride to inhibit reduction of THF to tributyl borate. BMS is soluble in most aprotic solvents.

tert-Butanesulfinamide is an organosulfur compound and a member of the class of sulfinamides. Both enantiomeric forms are commercially available and are used in asymmetric synthesis as chiral auxiliaries, often as chiral ammonia equivalents for the synthesis of amines. tert-Butanesulfinamide and the associated synthetic methodology was introduced in 1997 by Jonathan A. Ellman et al.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.
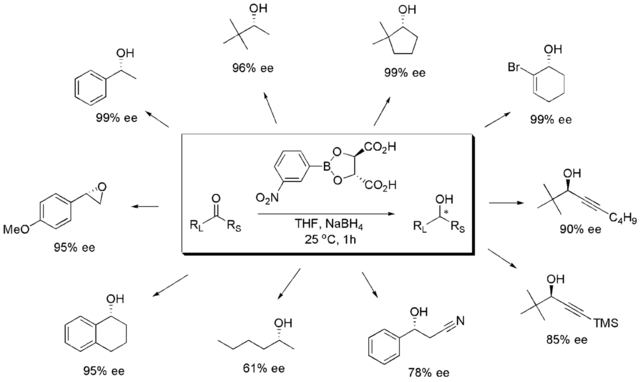
Enantioselective ketone reductions convert prochiral ketones into chiral, non-racemic alcohols and are used heavily for the synthesis of stereodefined alcohols.
Reductions with hydrosilanes are methods used for hydrogenation and hydrogenolysis of organic compounds. The approach is a subset of ionic hydrogenation. In this particular method, the substrate is treated with a hydrosilane and auxiliary reagent, often a strong acid, resulting in formal transfer of hydride from silicon to carbon. This style of reduction with hydrosilanes enjoys diverse if specialized applications.

The Enders SAMP/RAMP hydrazone alkylation reaction is an asymmetric carbon-carbon bond formation reaction facilitated by pyrrolidine chiral auxiliaries. It was pioneered by E. J. Corey and D. Enders in 1976, and was further developed by D. Enders and his group. This method is usually a three-step sequence. The first step is to form the hydrazone between (S)-1-amino-2-methoxymethylpyrrolidine (SAMP) or (R)-1-amino-2-methoxymethylpyrrolidine (RAMP) and a ketone or aldehyde. Afterwards, the hydrazone is deprotonated by lithium diisopropylamide (LDA) to form an azaenolate, which reacts with alkyl halides or other suitable electrophiles to give alkylated hydrazone species with the simultaneous generation of a new chiral center. Finally, the alkylated ketone or aldehyde can be regenerated by ozonolysis or hydrolysis.
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.
In organic chemistry, the Davis oxidation or Davis' oxaziridine oxidation refers to oxidations involving the use of the Davis reagent or other similar oxaziridine reagents. This reaction mainly refers to the generation of α-hydroxy carbonyl compounds (acyloins) from ketones or esters. The reaction is carried out in a basic environment to generate the corresponding enolate from the ketone or ester. This reaction has been shown to work for amides.