Related Research Articles

In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
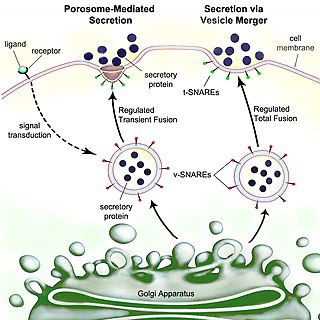
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the periplasmic space in gram-negative bacteria. Using cryo-electron microscopy it has been found that a much smaller periplasmic space is also present in gram-positive bacteria, between cell wall and the plasma membrane. The periplasm may constitute up to 40% of the total cell volume of gram-negative bacteria, but is a much smaller percentage in gram-positive bacteria.

Porins are beta barrel proteins that cross a cellular membrane and act as a pore, through which molecules can diffuse. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of gram-negative bacteria and some gram-positive mycobacteria, the outer membrane of mitochondria, and the outer chloroplast membrane.

The bacterial outer membrane is found in gram-negative bacteria. Its composition is distinct from that of the inner cytoplasmic cell membrane - among other things, the outer leaflet of the outer membrane of many gram-negative bacteria includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and in some bacteria such as E. coli it is linked to the cell's peptidoglycan by Braun's lipoprotein.

In protein structures, a beta barrel is a beta sheet composed of tandem repeats that twists and coils to form a closed toroidal structure in which the first strand is bonded to the last strand. Beta-strands in many beta-barrels are arranged in an antiparallel fashion. Beta barrel structures are named for resemblance to the barrels used to contain liquids. Most of them are water-soluble proteins and frequently bind hydrophobic ligands in the barrel center, as in lipocalins. Others span cell membranes and are commonly found in porins. Porin-like barrel structures are encoded by as many as 2–3% of the genes in Gram-negative bacteria. It has been shown that more than 600 proteins with various function contain the beta barrel structure.

Mitochondrial membrane transport proteins, also known as mitochondrial carrier proteins, are proteins which exist in the membranes of mitochondria. They serve to transport molecules and other factors, such as ions, into or out of the organelles. Mitochondria contain both an inner and outer membrane, separated by the inter-membrane space, or inner boundary membrane. The outer membrane is porous, whereas the inner membrane restricts the movement of all molecules. The two membranes also vary in membrane potential and pH. These factors play a role in the function of mitochondrial membrane transport proteins. There are 53 discovered human mitochondrial membrane transporters, with many others that are known to still need discovered.

General bacterial porins are a family of porin proteins from the outer membranes of Gram-negative bacteria. The porins act as molecular filters for hydrophilic compounds. They are responsible for the 'molecular sieve' properties of the outer membrane. Porins form large water-filled channels which allow the diffusion of hydrophilic molecules into the periplasmic space. Some porins form general diffusion channels that allow any solute up to a certain size to cross the membrane, while other porins are specific for one particular solute and contain a binding site for that solute inside the pores. As porins are the major outer membrane proteins, they also serve as receptor sites for the binding of phages and bacteriocins.
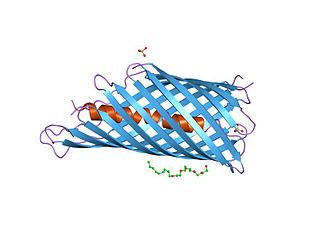
In molecular biology, an autotransporter domain is a structural domain found in some bacterial outer membrane proteins. The domain is always located at the C-terminal end of the protein and forms a beta-barrel structure. The barrel is oriented in the membrane such that the N-terminal portion of the protein, termed the passenger domain, is presented on the cell surface. These proteins are typically virulence factors, associated with infection or virulence in pathogenic bacteria.
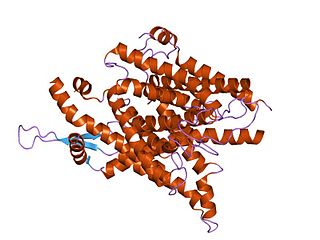
The SecY protein is the main transmembrane subunit of the bacterial Sec export pathway and of a protein-secreting ATPase complex, also known as a SecYEG translocon. Homologs of the SecYEG complex are found in eukaryotes and in archaea, where the subunit is known as Sec61α.

The fimbrial usher protein is involved in biogenesis of the pilus in Gram-negative bacteria. The biogenesis of some fimbriae requires a two-component assembly and transport system which is composed of a periplasmic chaperone and a pore-forming outer membrane protein which has been termed a molecular 'usher'; this is the chaperone-usher pathway.
SecD and SecF are prokaryotic protein export membrane proteins. They are a part of the larger multimeric protein export complex comprising SecA, D, E, F, G, Y, and YajC. SecD and SecF are required to maintain a proton motive force.

In molecular biology, trimeric autotransporter adhesins (TAAs), are proteins found on the outer membrane of Gram-negative bacteria. Bacteria use TAAs in order to infect their host cells via a process called cell adhesion. TAAs also go by another name, oligomeric coiled-coil adhesins, which is shortened to OCAs. In essence, they are virulence factors, factors that make the bacteria harmful and infective to the host organism.
Chaperone-usher fimbriae (CU) are linear, unbranching, outer-membrane pili secreted by gram-negative bacteria through the chaperone-usher system rather than through type IV secretion or extracellular nucleation systems. These fimbriae are built up out of modular pilus subunits, which are transported into the periplasm in a Sec dependent manner. Chaperone-usher secreted fimbriae are important pathogenicity factors facilitating host colonisation, localisation and biofilm formation in clinically important species such as uropathogenic Escherichia coli and Pseudomonas aeruginosa.

Outer membrane vesicles (OMVs) are vesicles released from the outer membranes of Gram-negative bacteria. While Gram-positive bacteria release vesicles as well those vesicles fall under the broader category of bacterial membrane vesicles (MVs). OMVs were the first MVs to be discovered, and are distinguished from outer inner membrane vesicles (OIMVS), which are gram-negative baterial vesicles containing portions of both the outer and inner bacterial membrane. Outer membrane vesicles were first discovered and characterized using transmission-electron microscopy by Indian Scientist Prof. Smriti Narayan Chatterjee and J. Das in 1966-67. OMVs are ascribed the functionality to provide a manner to communicate among themselves, with other microorganisms in their environment and with the host. These vesicles are involved in trafficking bacterial cell signaling biochemicals, which may include DNA, RNA, proteins, endotoxins and allied virulence molecules. This communication happens in microbial cultures in oceans, inside animals, plants and even inside the human body.
The type 2 secretion system is a type of protein secretion machinery found in various species of Gram-negative bacteria, including many human pathogens such as Pseudomonas aeruginosa and Vibrio cholerae. The type II secretion system is one of six protein secretory systems commonly found in Gram-negative bacteria, along with the type I, type III, and type IV secretion systems, as well as the chaperone/usher pathway, the autotransporter pathway/type V secretion system, and the type VI secretion system. Like these other systems, the type II secretion system enables the transport of cytoplasmic proteins across the lipid bilayers that make up the cell membranes of Gram-negative bacteria. Secretion of proteins and effector molecules out of the cell plays a critical role in signaling other cells and in the invasion and parasitism of host cells.

Resistance-nodulation-division (RND) family transporters are a category of bacterial efflux pumps, especially identified in Gram-negative bacteria and located in the cytoplasmic membrane, that actively transport substrates. The RND superfamily includes seven families: the heavy metal efflux (HME), the hydrophobe/amphiphile efflux-1, the nodulation factor exporter family (NFE), the SecDF protein-secretion accessory protein family, the hydrophobe/amphiphile efflux-2 family, the eukaryotic sterol homeostasis family, and the hydrophobe/amphiphile efflux-3 family. These RND systems are involved in maintaining homeostasis of the cell, removal of toxic compounds, and export of virulence determinants. They have a broad substrate spectrum and can lead to the diminished activity of unrelated drug classes if over-expressed. The first reports of drug resistant bacterial infections were reported in the 1940s after the first mass production of antibiotics. Most of the RND superfamily transport systems are made of large polypeptide chains. RND proteins exist primarily in gram-negative bacteria but can also be found in gram-positive bacteria, archaea, and eukaryotes.

Bacterial secretion systems are protein complexes present on the cell membranes of bacteria for secretion of substances. Specifically, they are the cellular devices used by pathogenic bacteria to secrete their virulence factors to invade the host cells. They can be classified into different types based on their specific structure, composition and activity. Generally, proteins can be secreted through two different processes. One process is a one-step mechanism in which proteins from the cytoplasm of bacteria are transported and delivered directly through the cell membrane into the host cell. Another involves a two-step activity in which the proteins are first transported out of the inner cell membrane, then deposited in the periplasm, and finally through the outer cell membrane into the host cell.
The bacterial type IV secretion system, also known as the type IV secretion system or the T4SS, is a secretion protein complex found in gram negative bacteria, gram positive bacteria, and archaea. It is able to transport proteins and DNA across the cell membrane. The type IV secretion system is just one of many bacterial secretion systems. Type IV secretion systems are related to conjugation machinery which generally involve a single-step secretion system and the use of a pilus. Type IV secretion systems are used for conjugation, DNA exchange with the extracellular space, and for delivering proteins to target cells. The type IV secretion system is divided into type IVA and type IVB based on genetic ancestry.

Darobactin is an experimental antibiotic compound that may be effective against Gram-negative bacteria. If it can be developed into a human-compatible form it would be the first to come from an animal microbiome.
References
- ↑ Walther DM, Rapaport D, Tommassen J (September 2009). "Biogenesis of beta-barrel membrane proteins in bacteria and eukaryotes: evolutionary conservation and divergence". Cellular and Molecular Life Sciences. 66 (17): 2789–804. doi:10.1007/s00018-009-0029-z. PMC 2724633 . PMID 19399587.
- ↑ Habib SJ, Waizenegger T, Niewienda A, Paschen SA, Neupert W, Rapaport D (January 2007). "The N-terminal domain of Tob55 has a receptor-like function in the biogenesis of mitochondrial beta-barrel proteins". The Journal of Cell Biology. 176 (1): 77–88. doi:10.1083/jcb.200602050. PMC 2063629 . PMID 17190789.
- ↑ Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR (March 2009). "Membrane protein architects: the role of the BAM complex in outer membrane protein assembly". Nature Reviews. Microbiology. 7 (3): 206–14. doi: 10.1038/nrmicro2069 . PMID 19182809.
- ↑ Hagan CL, Silhavy TJ, Kahne D (2011). "β-Barrel membrane protein assembly by the Bam complex". Annual Review of Biochemistry. 80: 189–210. doi:10.1146/annurev-biochem-061408-144611. PMID 21370981.
- ↑ Rigel NW, Silhavy TJ (April 2012). "Making a beta-barrel: assembly of outer membrane proteins in Gram-negative bacteria". Current Opinion in Microbiology. 15 (2): 189–93. doi:10.1016/j.mib.2011.12.007. PMC 3320693 . PMID 22221898.
- ↑ Jiang JH, Tong J, Tan KS, Gabriel K (2012). "From evolution to pathogenesis: the link between β-barrel assembly machineries in the outer membrane of mitochondria and gram-negative bacteria". International Journal of Molecular Sciences. 13 (7): 8038–50. doi: 10.3390/ijms13078038 . PMC 3430219 . PMID 22942688.
- ↑ Tommassen J (September 2010). "Assembly of outer-membrane proteins in bacteria and mitochondria". Microbiology. Reading, England. 156 (Pt 9): 2587–2596. doi: 10.1099/mic.0.042689-0 . PMID 20616105.
- ↑ Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N (August 2009). "Importing mitochondrial proteins: machineries and mechanisms". Cell. 138 (4): 628–44. doi:10.1016/j.cell.2009.08.005. PMC 4099469 . PMID 19703392.
- ↑ Webb CT, Heinz E, Lithgow T (December 2012). "Evolution of the β-barrel assembly machinery". Trends in Microbiology. 20 (12): 612–20. doi:10.1016/j.tim.2012.08.006. PMID 22959613.
- ↑ Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R (July 2010). "A new bioinformatics analysis tools framework at EMBL-EBI". Nucleic Acids Research. 38 (Web Server issue): W695–9. doi:10.1093/nar/gkq313. PMC 2896090 . PMID 20439314.
- ↑ Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D (April 2005). "Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli". Cell. 121 (2): 235–45. doi: 10.1016/j.cell.2005.02.015 . PMID 15851030.
- ↑ Noinaj N, Kuszak AJ, Gumbart JC, Lukacik P, Chang H, Easley NC, Lithgow T, Buchanan SK (September 2013). "Structural insight into the biogenesis of β-barrel membrane proteins". Nature. 501 (7467): 385–90. doi:10.1038/nature12521. PMC 3779476 . PMID 23995689.