
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.

In surface science, surface energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energetically favorable than the bulk of the material, otherwise there would be a driving force for surfaces to be created, removing the bulk of the material by sublimation. The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is the work required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. There is "excess energy" as a result of the now-incomplete, unrealized bonding between the two created surfaces.
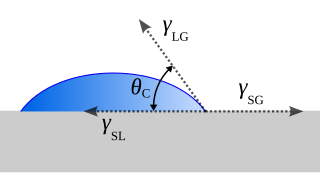
In fluid mechanics, dewetting is one of the processes that can occur at a solid–liquid, solid–solid or liquid–liquid interface. Generally, dewetting describes the process of retraction of a fluid from a non-wettable surface it was forced to cover. The opposite process—spreading of a liquid on a substrate—is called wetting. The factor determining the spontaneous spreading and dewetting for a drop of liquid placed on a solid substrate with ambient gas, is the so-called spreading coefficient S:

A Langmuir–Blodgett trough is an item of laboratory apparatus that is used to compress monolayers of molecules on the surface of a given subphase and to measure surface phenomena due to this compression. It can also be used to deposit single or multiple monolayers on a solid substrate.

The contact angle is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interface at their intersection. It quantifies the wettability of a solid surface by a liquid via the Young equation.

A Langmuir–Blodgett (LB) film is a nanostructured system formed when Langmuir films—or Langmuir monolayers (LM)—are transferred from the liquid-gas interface to solid supports during the vertical passage of the support through the monolayers. LB films can contain one or more monolayers of an organic material, deposited from the surface of a liquid onto a solid by immersing the solid substrate into the liquid. A monolayer is adsorbed homogeneously with each immersion or emersion step, thus films with very accurate thickness can be formed. This thickness is accurate because the thickness of each monolayer is known and can therefore be added to find the total thickness of a Langmuir–Blodgett film.

Plasma cleaning is the removal of impurities and contaminants from surfaces through the use of an energetic plasma or dielectric barrier discharge (DBD) plasma created from gaseous species. Gases such as argon and oxygen, as well as mixtures such as air and hydrogen/nitrogen are used. The plasma is created by using high frequency voltages to ionise the low pressure gas, although atmospheric pressure plasmas are now also common.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.

In materials science, chemical force microscopy (CFM) is a variation of atomic force microscopy (AFM) which has become a versatile tool for characterization of materials surfaces. With AFM, structural morphology is probed using simple tapping or contact modes that utilize van der Waals interactions between tip and sample to maintain a constant probe deflection amplitude or maintain height while measuring tip deflection. CFM, on the other hand, uses chemical interactions between functionalized probe tip and sample. Choice chemistry is typically gold-coated tip and surface with R−SH thiols attached, R being the functional groups of interest. CFM enables the ability to determine the chemical nature of surfaces, irrespective of their specific morphology, and facilitates studies of basic chemical bonding enthalpy and surface energy. Typically, CFM is limited by thermal vibrations within the cantilever holding the probe. This limits force measurement resolution to ~1 pN, which is still very suitable considering weak COOH/CH3 interactions are ~20 pN per pair. Hydrophobicity is used as the primary example throughout this consideration of CFM, but certainly any type of bonding can be probed with this method.
Biofilm formation occurs when free floating microorganisms attach themselves to a surface. Although there are some beneficial uses of biofilms, they are generally considered undesirable, and means of biofilm prevention have been developed. Biofilms secrete extracellular polymeric substance that provides a structural matrix and facilitates adhesion for the microorganisms; the means of prevention have thus concentrated largely on two areas: killing the microbes that form the film, or preventing the adhesion of the microbes to a surface. Because biofilms protect the bacteria, they are often more resistant to traditional antimicrobial treatments, making them a serious health risk. For example, there are more than one million cases of catheter-associated urinary tract infections (CAUTI) reported each year, many of which can be attributed to bacterial biofilms. There is much research into the prevention of biofilms.
Adsorption is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without surface penetration occurring. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and as such they adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.
Adsorption is the adhesion of ions or molecules onto the surface of another phase. Adsorption may occur via physisorption and chemisorption. Ions and molecules can adsorb to many types of surfaces including polymer surfaces. A polymer is a large molecule composed of repeating subunits bound together by covalent bonds. In dilute solution, polymers form globule structures. When a polymer adsorbs to a surface that it interacts favorably with, the globule is essentially squashed, and the polymer has a pancake structure.

Biomaterials are materials that are used in contact with biological systems. Biocompatibility and applicability of surface modification with current uses of metallic, polymeric and ceramic biomaterials allow alteration of properties to enhance performance in a biological environment while retaining bulk properties of the desired device.
Polymeric materials have widespread application due to their versatile characteristics, cost-effectiveness, and highly tailored production. The science of polymer synthesis allows for excellent control over the properties of a bulk polymer sample. However, surface interactions of polymer substrates are an essential area of study in biotechnology, nanotechnology, and in all forms of coating applications. In these cases, the surface characteristics of the polymer and material, and the resulting forces between them largely determine its utility and reliability. In biomedical applications for example, the bodily response to foreign material, and thus biocompatibility, is governed by surface interactions. In addition, surface science is integral part of the formulation, manufacturing, and application of coatings.

Photolithography is a process in removing select portions of thin films used in microfabrication. Microfabrication is the production of parts on the micro- and nano- scale, typically on the surface of silicon wafers, for the production of integrated circuits, microelectromechanical systems (MEMS), solar cells, and other devices. Photolithography makes this process possible through the combined use of hexamethyldisilazane (HMDS), photoresist, spin coating, photomask, an exposure system and other various chemicals. By carefully manipulating these factors it is possible to create nearly any geometry microstructure on the surface of a silicon wafer. The chemical interaction between all the different components and the surface of the silicon wafer makes photolithography an interesting chemistry problem. Current engineering has been able to create features on the surface of silicon wafers between 1 and 100 μm.

Bovine submaxillary mucin (BSM) coatings are a surface treatment provided to biomaterials intended to reduce the growth of disadvantageous bacteria and fungi such as S. epidermidis, E. coli, and Candida albicans. BSM is a substance extracted from the fresh salivary glands of cows. It exhibits unique physical properties, such as high molecular weight and amphiphilicity, that allow it to be used for many biomedical applications.
The surface chemistry of paper is responsible for many important paper properties, such as gloss, waterproofing, and printability. Many components are used in the paper-making process that affect the surface.
Ultra-low fouling is a rating of a surface's ability to shed potential contamination. Surfaces are prone to contamination, which is a phenomenon known as fouling. Unwanted adsorbates caused by fouling change the properties of a surface, which is often counter-productive to the function of that surface. Consequently, a necessity for anti-fouling surfaces has arisen in many fields: blocked pipes inhibit factory productivity, biofouling increases fuel consumption on ships, medical devices must be kept sanitary, etc. Although chemical fouling inhibitors, metallic coatings, and cleaning processes can be used to reduce fouling, non-toxic surfaces with anti-fouling properties are ideal for fouling prevention. To be considered effective, an ultra-low fouling surface must be able to repel and withstand the accumulation of detrimental aggregates down to less than 5 ng/cm2. A recent surge of research has been conducted to create these surfaces in order to benefit the biological, nautical, mechanical, and medical fields.

Self-healing hydrogels are a specialized type of polymer hydrogel. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are synthesized from hydrophilic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhance the hydrogel's ability to absorb large amounts of water via hydrogen bonding. As a result, hydrogels, self-healing alike, develop characteristic firm yet elastic mechanical properties. Self-healing refers to the spontaneous formation of new bonds when old bonds are broken within a material. The structure of the hydrogel along with electrostatic attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties have motivated the research and development of self-healing hydrogels in fields such as reconstructive tissue engineering as scaffolding, as well as use in passive and preventive applications.
Self-cleaning surfaces are a class of materials with the inherent ability to remove any debris or bacteria from their surfaces in a variety of ways. The self-cleaning functionality of these surfaces are commonly inspired by natural phenomena observed in lotus leaves, gecko feet, and water striders to name a few. The majority of self-cleaning surfaces can be placed into three categories:
- superhydrophobic
- superhydrophilic
- photocatalytic.






















