Related Research Articles

Covellite is a rare copper sulfide mineral with the formula CuS. This indigo blue mineral is commonly a secondary mineral in limited abundance and although it is not an important ore of copper itself, it is well known to mineral collectors.

Torbernite, also known as chalcolite, is a relatively common mineral with the chemical formula Cu[(UO2)(PO4)]2(H2O)12. It is a radioactive, hydrated green copper uranyl phosphate, found in granites and other uranium-bearing deposits as a secondary mineral. The chemical formula of torbernite is similar to that of autunite in which a Cu2+ cation replaces a Ca2+ cation. Torbernite tends to dehydrate to metatorbernite with the sum formula Cu[(UO2)(PO4)]2(H2O)8.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.
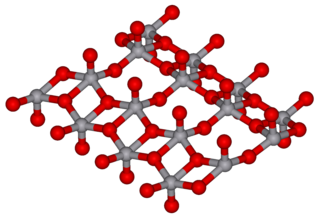
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.
Ziesite is a copper vanadate mineral with formula: β-Cu2V2O7. It was discovered in 1980 as monoclinic crystals occurring as volcanic sublimates around fumaroles in the crater of the Izalco Volcano, El Salvador. It is named after Emanuel George Zies (1883–1981), an American geochemist who studied Izalco in the 1930s.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".

Izalco is an active stratovolcano on the side of the Santa Ana Volcano, which is located in western El Salvador. It is situated on the southern flank of the Santa Ana volcano. Izalco erupted almost continuously from 1770 to 1958 earning it the nickname of "Lighthouse of the Pacific", and experienced a flank eruption in 1966. During an eruption in 1926, the village of Matazano was buried and 56 people were killed. The volcano erupted on highly arable land which was used for the production of coffee, cacao, and sugar cane.
Lyonsite (Cu3Fe+34(VO4)6) is a rare black vanadate mineral that is opaque with a metallic lustre. It crystallizes in the orthorhombic crystal system. Lyonsite often occurs as small tabular typically well formed crystals. Lyonsite has a good cleavage and a dark gray streak.
Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. Both minerals and synthetic materials comprise these compounds. Some copper sulfides are economically important ores.

Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S. Samples are typically black.

Bararite is a natural form of ammonium fluorosilicate (also known as hexafluorosilicate or fluosilicate). It has chemical formula (NH4)2SiF6 and trigonal crystal structure. This mineral was once classified as part of cryptohalite. Bararite is named after the place where it was first described, Barari, India. It is found at the fumaroles of volcanoes (Vesuvius, Italy), over burning coal seams (Barari, India), and in burning piles of anthracite (Pennsylvania, U.S.). It is a sublimation product that forms with cryptohalite, sal ammoniac, and native sulfur.

Abramovite is a very rare mineral from the sulfides and sulfosalt categories. It has the chemical formula Pb2SnInBiS7. It occurs as tiny elongated lamellar-shaped crystals, up 1 mm × 0.2 mm in size, and is characterized by its non-commensurate structure.

Delafossite is a copper iron oxide mineral with formula CuFeO2 or Cu1+Fe3+O2. It is a member of the delafossite mineral group, which has the general formula ABO2, a group characterized by sheets of linearly coordinated A cations stacked between edge-shared octahedral layers (BO6). Delafossite, along with other minerals of the ABO2 group, is known for its wide range of electrical properties, its conductivity varying from insulating to metallic. Delafossite is usually a secondary mineral that crystallizes in association with oxidized copper and rarely occurs as a primary mineral.
Fingerite is a copper vanadate mineral with formula: β-Cu2V2O5. It was discovered as triclinic crystals occurring as volcanic sublimates around fumaroles in the crater of the Izalco Volcano, El Salvador.
Chrysothallite is a rare thallium-bearing chloride mineral with the formula K6Cu6Tl3+Cl17(OH)4•H2O. Chrysothallite is unique in being only the second mineral with essential trivalent thallium, a feature shared with natural thallium(III) oxide, avicennite. Another examples of natural thallium chlorides are steropesite, Tl3BiCl6, and lafossaite, TlCl. Chrysothallite is one of numerous fumarolic minerals discovered among fumarolic sites of the Tolbachik volcano, Kamchatka, Russia The mineral is named in allusion to its colour and thallium content.
Kainotropite is a rare vanadate mineral with the formula Cu4FeO2(V2O7)(VO4). It contains trivalent iron. It is one of many fumarolic minerals discovered on the Tolbachik volcano. The name of its parental fumarole is "Yadovitaya", which means poisonous.
Wulffite is an alkali copper sulfate mineral with the chemical formula K3NaCu4O2(SO4)4, in the sulfate category of minerals. It was recently discovered in Kamchatka, Russia at the Tolbachik volcano in 2012. It was named for Russian crystallographer Georgiy Viktorovich Wulff, a renowned expert who furthered X-ray diffraction and interference. Wullfite shares many properties with parawulffite, which was found in the same area just with slightly different chemical composition.

Euchlorine (KNaCu3(SO4)3O) is a rare emerald-green colored sulfate mineral found naturally occurring as a sublimate in fumaroles around volcanic eruptions. It was first discovered in fumaroles of the 1868 eruption at Mount Vesuvius in Campania, Italy by Arcangelo Scacchi. The name 'euchlorine' comes from the Greek word εΰχλωρος meaning "pale green" in reference to the mineral's color, other reported spellings include euclorina, euchlorin, and euchlorite.

Fumarole minerals are minerals which are deposited by fumarole exhalations. They form when gases and compounds desublimate or precipitate out of condensates, forming mineral deposits. They are mostly associated with volcanoes following deposition from volcanic gas during an eruption or discharge from a volcanic vent or fumarole, but have been encountered on burning coal deposits as well. They can be black or multicoloured and are often unstable upon exposure to the atmosphere.
Aleutite is both a vanadate and arsenate mineral but it can also be considered as a natural salt-inclusion phase that was first discovered at Second scoria cone of the Great Fissure Tolbachik eruption in the summer of 2015 in Kamchatka, Russia. Aleutite is a fumarolic mineral found with many other newly discovered minerals at this location. It gained the name from the Aleuts, the ethnic group who are the original inhabitants living on the Commander Islands, Aleutsky District, Kamchatka Krai. This mineral is very brittle and has a dark red color. Aleutite is a new structure type, the structure was refined as a 2-component twin, the twin ratio equals (0.955:0.045).
References
- ↑ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi: 10.1180/mgm.2021.43 . S2CID 235729616.
- ↑ Blossite on Mindat.org
- ↑ Blossite data on Webmineral
- ↑ Blossite in the Handbook of Mineralogy
- 1 2 3 4 Krivovichev, S.V., Filatov, S.K., and Cherepansky, P.N. (2005) Crystal structure of γ-Cu2V2O7 and its comparison to blossite (α-Cu2V2O7) and ziesite (β-Cu2V2O7). Canadian Mineralogist, 43, 671–677
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Robinson, P.D., Hughes, J.M., and Malinconico, M.L. (1987) Blossite, α-Cu_2^2+V_2^5+O7, a new fumarolic sublimate from Izalco volcano, El Salvador. American Mineralogist, 72, 397–400
- ↑ Stoiber, R.E., Rose, W.I., Lange, I.M., and Birnie, R.W. (1975) The cooling of Izalco volcano (El Salvador) 1964–1974. GeologischesJahrbuch, 13, 193–205.
- ↑ Stoiber, R.E., and Rose, W.I. (1974) Fumarole incrustations at active Central American Volcanoes.GeochimaetCosmochimicaActa, 38, 495–516.