Related Research Articles

Agarose is a polysaccharide, generally extracted from certain red seaweed. It is a linear polymer made up of the repeating unit of agarobiose, which is a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose. Agarose is one of the two principal components of agar, and is purified from agar by removing agar's other component, agaropectin.

Gel electrophoresis is a method for separation and analysis of macromolecules and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.

Molecular biology is the branch of biology that concerns the molecular basis of biological activity in and between cells, including molecular synthesis, modification, mechanisms and interactions.
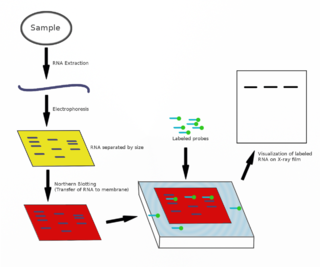
The northern blot, or RNA blot, is a technique used in molecular biology research to study gene expression by detection of RNA in a sample.

A Southern blot is a method used in molecular biology for detection of a specific DNA sequence in DNA samples. Southern blotting combines transfer of electrophoresis-separated DNA fragments to a filter membrane and subsequent fragment detection by probe hybridization.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

The western blot, or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.
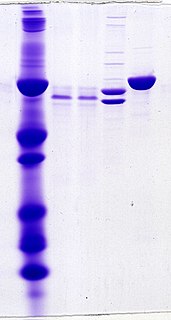
Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium: SDS polyacrylamide gel electrophoresis, free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each method has many variations with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting immunoblotting to give additional information about a specific protein. Because of practical limitations, protein electrophoresis is generally not suited as a preparative method.
A blot, in molecular biology and genetics, is a method of transferring proteins, DNA or RNA onto a carrier. In many instances, this is done after a gel electrophoresis, transferring the molecules from the gel onto the blotting membrane, and other times adding the samples directly onto the membrane. After the blotting, the transferred proteins, DNA or RNA are then visualized by colorant staining, autoradiographic visualization of radiolabelled molecules, or specific labelling of some proteins or nucleic acids. The latter is done with antibodies or hybridization probes that bind only to some molecules of the blot and have an enzyme joined to them. After proper washing, this enzymatic activity is visualized by incubation with proper reactive, rendering either a colored deposit on the blot or a chemiluminescent reaction which is registered by photographic film.
Biomedicine is a branch of medical science that applies biological and physiological principles to clinical practice. Biomedicine stresses standardized, evidence-based treatment validated through biological research, with treatment administered via formally trained doctors, nurses, and other such licensed practitioners.
Affinity chromatography is a method of separating biochemical mixture based on a highly specific interaction between antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid. It is a type of chromatographic laboratory technique used for purifying biological molecules within a mixture by exploiting molecular properties, e.g. protein can be eluted by ligand solution. Biological macromolecules, such as enzymes and other proteins, interact with other molecules with high specificity through several different types of bonds and interaction. Such interactions include hydrogen bonding, ionic interaction, disulfide bridges, hydrophobic interaction, and more. The high selectivity of affinity chromatography is caused by allowing the desired molecule to interact with the stationary phase and be bound within the column in order to be separated from the undesired material which will not interact and elute first. The molecules no longer needed are first washed away with a buffer while the desired proteins are let go in the presence of the eluting solvent. This process creates a competitive interaction between the desired protein and the immobilized stationary molecules, which eventually lets the now highly purified proteins be released.
Protein methods are the techniques used to study proteins. There are experimental methods for studying proteins. Computational methods typically use computer programs to analyze proteins. However, many experimental methods require computational analysis of the raw data.

A dot blot is a technique in molecular biology used to detect proteins. It represents a simplification of the western blot method, with the exception that the proteins to be detected are not first separated by electrophoresis. Instead, the sample is applied directly on a membrane in a single spot, and the blotting procedure is performed.
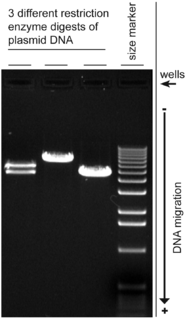
A molecular-weight size marker, also referred to as a protein ladder, DNA ladder, or RNA ladder, is a set of standards that are used to identify the approximate size of a molecule run on a gel during electrophoresis, using the principle that molecular weight is inversely proportional to migration rate through a gel matrix. Therefore, when used in gel electrophoresis, markers effectively provide a logarithmic scale by which to estimate the size of the other fragments.
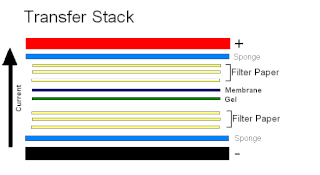
Electroblotting is a method in molecular biology/biochemistry/immunogenetics to transfer proteins or nucleic acids onto a membrane by using PVDF or nitrocellulose, after gel electrophoresis. The protein or nucleic acid can then be further analyzed using probes such as specific antibodies, ligands like lectins, or stains. This method can be used with all polyacrylamide and agarose gels. An alternative technique for transferring proteins from a gel is capillary blotting.

Affinity electrophoresis is a general name for many analytical methods used in biochemistry and biotechnology. Both qualitative and quantitative information may be obtained through affinity electrophoresis. The methods include the so-called electrophoretic mobility shift assay, charge shift electrophoresis and affinity capillary electrophoresis. The methods are based on changes in the electrophoretic pattern of molecules through biospecific interaction or complex formation. The interaction or binding of a molecule, charged or uncharged, will normally change the electrophoretic properties of a molecule. Membrane proteins may be identified by a shift in mobility induced by a charged detergent. Nucleic acids or nucleic acid fragments may be characterized by their affinity to other molecules. The methods have been used for estimation of binding constants, as for instance in lectin affinity electrophoresis or characterization of molecules with specific features like glycan content or ligand binding. For enzymes and other ligand-binding proteins, one-dimensional electrophoresis similar to counter electrophoresis or to "rocket immunoelectrophoresis", affinity electrophoresis may be used as an alternative quantification of the protein. Some of the methods are similar to affinity chromatography by use of immobilized ligands.
The eastern blot, or eastern blotting, is a biochemical technique used to analyze protein post-translational modifications (PTM) including the addition of lipids, phosphates, and glycoconjugates. It is most often used to detect carbohydrate epitopes. Thus, eastern blot can be considered an extension of the biochemical technique of western blot. Multiple techniques have been described by the term "eastern blot(ting)", most use prospo protein blotted from SDS-PAGE gel on to a PVDF or nitrocellulose membrane. Transferred proteins are analyzed for post-translational modifications using probes that may detect lipids, carbohydrate, phosphorylation or any other protein modification. Eastern blotting should be used to refer to methods that detect their targets through specific interaction of the PTM and the probe, distinguishing them from a standard far-western blot. In principle, eastern blotting is similar to lectin blotting.
The northwestern blot, also known as the northwestern assay, is a hybrid analytical technique of the western blot and the northern blot, and is used in molecular biology to detect interactions between RNA and proteins. A related technique, the western blot, is used to detect a protein of interest that involves transferring proteins that are separated by gel electrophoresis onto a nitrocellulose membrane. A colored precipitate clusters along the band on the membrane containing a particular target protein. A northern blot is a similar analytical technique that, instead of detecting a protein of interest, is used to study gene expression by detection of RNA on a similar membrane. The northwestern blot combines the two techniques, and specifically involves the identification of labeled RNA that interact with proteins that are immobilized on a similar nitrocellulose membrane.

SDS-PAGE, the use of sodium dodecyl sulfate and polyacrylamide gel largely eliminates the influence of the structure and charge, and proteins are separated solely based on polypeptide chain length. It uses sodium dodecyl sulfate (SDS) molecules to help identify and isolate protein molecules.
References
- 1 2 Biji T. Kurien, ed. (2009). Protein Blotting and Detection. R. Hal Scofield. New York: Humana Press. ISBN 978-1934115732.
- ↑ Merril, Carl R.; Washart, Karen M. (1998). Gel Electrophoresis of Proteins (PDF). Oxford University Press. p. 71.
| This molecular biology article is a stub. You can help Wikipedia by expanding it. |