
Leukemia is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called blasts or leukemia cells. Symptoms may include bleeding and bruising, bone pain, fatigue, fever, and an increased risk of infections. These symptoms occur due to a lack of normal blood cells. Diagnosis is typically made by blood tests or bone marrow biopsy.
Diarrhea, also spelled diarrhoea or diarrhœa, is the condition of having at least three loose, liquid, or watery bowel movements in a day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin with loss of the normal stretchiness of the skin and irritable behaviour. This can progress to decreased urination, loss of skin color, a fast heart rate, and a decrease in responsiveness as it becomes more severe. Loose but non-watery stools in babies who are exclusively breastfed, however, are normal.

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
Food intolerance is a detrimental reaction, often delayed, to a food, beverage, food additive, or compound found in foods that produces symptoms in one or more body organs and systems, but generally refers to reactions other than food allergy. Food hypersensitivity is used to refer broadly to both food intolerances and food allergies.

Cetuximab, sold under the brand name Erbitux, is an epidermal growth factor receptor (EGFR) inhibitor medication used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness (efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. These research procedures are designed for the prevention, treatment, diagnosis or understanding of disease symptoms.

Nilotinib, sold under the brand name Tasigna marketed worldwide by Novartis, is a medication used to treat chronic myelogenous leukemia (CML) which has the Philadelphia chromosome. It may be used both in initial cases of chronic phase CML as well as in accelerated and chronic phase CML that has not responded to imatinib. It is taken by mouth.

Dasatinib, sold under the brand name Sprycel among others, is a targeted therapy medication used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL). Specifically it is used to treat cases that are Philadelphia chromosome-positive (Ph+). It is taken by mouth.
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.

Sucrose intolerance or genetic sucrase-isomaltase deficiency (GSID) is the condition in which sucrase-isomaltase, an enzyme needed for proper metabolism of sucrose (sugar) and starch, is not produced or the enzyme produced is either partially functional or non-functional in the small intestine. All GSID patients lack fully functional sucrase, while the isomaltase activity can vary from minimal functionality to almost normal activity. The presence of residual isomaltase activity may explain why some GSID patients are better able to tolerate starch in their diet than others with GSID.

Plus Therapeutics, Inc. is a clinical-stage pharmaceutical company developing innovative, targeted radiotherapeutics for adults and children with rare and difficult-to-treat cancers. The company is headquartered in Austin, Texas, United States.
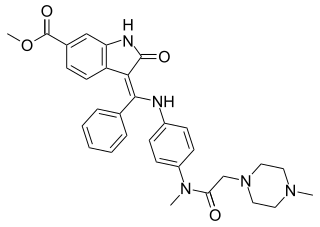
Nintedanib, sold under the brand names Ofev and Vargatef, is an oral medication used for the treatment of idiopathic pulmonary fibrosis and along with other medications for some types of non-small-cell lung cancer.

The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.
Demcizumab is a humanized monoclonal antibody which is used to treat patients with pancreatic cancer or non-small cell lung cancer. Demcizumab has completed phase 1 trials and is currently undergoing phase 2 trials. Demcizumab was developed by OncoMed Pharmaceuticals in collaboration with Celgene.
Margetuximab, sold under the brand name Margenza, is a chimeric IgG monoclonal antibody medication against HER2 used for the treatment of cancer.

Sotorasib, sold under the brand names Lumakras and Lumykras, is an anti-cancer medication used to treat non-small-cell lung cancer. It targets a specific mutation, G12C, in the protein K-Ras encoded by gene KRAS which is responsible for various forms of cancer. Sotorasib is an inhibitor of the RAS GTPase family.
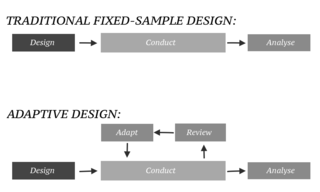
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial endpoint. This is in contrast to traditional single-arm clinical trials or randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA provides not only a summary of different adaptive designs, but also comprehensive information on adaptive design planning, conduct, analysis and reporting.
Infigratinib is an kinase inhibitor in development for the treatment of achondroplasia and hypochondroplasia.














