
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.

The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elastic medium. At 20 °C (68 °F), the speed of sound in air is about 343 m/s, or one km in 2.91 s or one mile in 4.69 s. It depends strongly on temperature as well as the medium through which a sound wave is propagating. At 0 °C (32 °F), the speed of sound in air is about 331 m/s. More simply, the speed of sound is how fast vibrations travel.

In physics and fluid mechanics, a boundary layer is the thin layer of fluid in the immediate vicinity of a bounding surface formed by the fluid flowing along the surface. The fluid's interaction with the wall induces a no-slip boundary condition. The flow velocity then monotonically increases above the surface until it returns to the bulk flow velocity. The thin layer consisting of fluid whose velocity has not yet returned to the bulk flow velocity is called the velocity boundary layer.
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.
Photoacoustic spectroscopy is the measurement of the effect of absorbed electromagnetic energy on matter by means of acoustic detection. The discovery of the photoacoustic effect dates to 1880 when Alexander Graham Bell showed that thin discs emitted sound when exposed to a beam of sunlight that was rapidly interrupted with a rotating slotted disk. The absorbed energy from the light causes local heating, generating a thermal expansion which creates a pressure wave or sound. Later Bell showed that materials exposed to the non-visible portions of the solar spectrum can also produce sounds.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field. This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increased proportionally to the strength of the external magnetic field. Notably, the resonance frequency of each NMR-active nucleus depends on its chemical environment. As a result, NMR spectra provide information about individual functional groups present in the sample, as well as about connections between nearby nuclei in the same molecule. As the NMR spectra are unique or highly characteristic to individual compounds and functional groups, NMR spectroscopy is one of the most important methods to identify molecular structures, particularly of organic compounds.
An acoustic interferometer is an instrument that uses interferometry to measure the physical characteristics of sound waves in a gas or liquid. It may be used to measure velocity, wavelength, absorption, or impedance of the sound waves. The principle of operation is that a vibrating crystal creates ultrasonic waves that are radiated into the medium being analyzed. The waves strike a reflector placed parallel to the crystal. The waves are then reflected back to the source and measured.

A bubble is a globule of a gas substance in a liquid. In the opposite case, a globule of a liquid in a gas, is called a drop. Due to the Marangoni effect, bubbles may remain intact when they reach the surface of the immersive substance.
The hot chocolate effect is a phenomenon of wave mechanics in which the pitch heard from tapping a cup of hot liquid rises after the addition of a soluble powder. The effect is thought to happen because upon initial stirring, entrained gas bubbles reduce the speed of sound in the liquid, lowering the frequency. As the bubbles clear, sound travels faster in the liquid and the frequency increases.
Electrophoretic light scattering is based on dynamic light scattering. The frequency shift or phase shift of an incident laser beam depends on the dispersed particles mobility. With dynamic light scattering, Brownian motion causes particle motion. With electrophoretic light scattering, oscillating electric field performs this function.
Acoustic resonance spectroscopy (ARS) is a method of spectroscopy in the acoustic region, primarily the sonic and ultrasonic regions. ARS is typically much more rapid than HPLC and NIR. It is non destructive and requires no sample preparation as the sampling waveguide can simply be pushed into a sample powder/liquid or in contact with a solid sample. To date, the AR spectrometer has successfully differentiated and quantified sample analytes in various forms;. It has been used to measure and monitor the progression of chemical reactions, such as the setting and hardening of concrete from cement paste to solid. Acoustic spectrometry has also been used to measure the volume fraction of colloids in a dispersion medium, as well as for the investigation of physical properties of colloidal dispersions, such as aggregation and particle size distribution. Typically, these experiments are carried out with sinusoidal excitation signals and the experimental observation of signal attenuation. From a comparison of theoretical attenuation to experimental observation, the particle size distribution and aggregation phenomena are inferred.

An acoustic metamaterial, sonic crystal, or phononic crystal is a material designed to control, direct, and manipulate sound waves or phonons in gases, liquids, and solids. Sound wave control is accomplished through manipulating parameters such as the bulk modulus β, density ρ, and chirality. They can be engineered to either transmit, or trap and amplify sound waves at certain frequencies. In the latter case, the material is an acoustic resonator.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). The original application of NMR to condensed matter physics is nowadays mostly devoted to strongly correlated electron systems. It reveals large manybody couplings by fast broadband detection and it should not to be confused with solid state NMR, which aims at removing the effect of the same couplings by Magic Angle Spinning techniques.
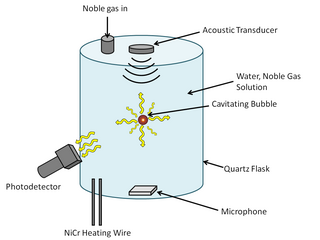
Sonoluminescence is a phenomenon that occurs when a small gas bubble is acoustically suspended and periodically driven in a liquid solution at ultrasonic frequencies, resulting in bubble collapse, cavitation, and light emission. The thermal energy that is released from the bubble collapse is so great that it can cause weak light emission. The mechanism of the light emission remains uncertain, but some of the current theories, which are categorized under either thermal or electrical processes, are Bremsstrahlung radiation, argon rectification hypothesis, and hot spot. Some researchers are beginning to favor thermal process explanations as temperature differences have consistently been observed with different methods of spectral analysis. In order to understand the light emission mechanism, it is important to know what is happening in the bubble's interior and at the bubble's surface.
Channel sounding is a technique that evaluates a radio environment for wireless communication, especially MIMO systems. Because of the effect of terrain and obstacles, wireless signals propagate in multiple paths. To minimize or use the multipath effect, engineers use channel sounding to process the multidimensional spatial–temporal signal and estimate channel characteristics. This helps simulate and design wireless systems.
Bjerknes forces are translational forces on bubbles in a sound wave. The phenomenon is a type of acoustic radiation force. Primary Bjerknes forces are caused by an external sound field; secondary Bjerknes forces are attractive or repulsive forces between pairs of bubbles in the same sound field caused by the pressure field generated by each bubble volume's oscillations. They were first described by Vilhelm Bjerknes in his 1906 Fields of Force.
Nuclear acoustic resonance is a phenomenon closely related to nuclear magnetic resonance. It involves utilizing ultrasound and ultrasonic acoustic waves of frequencies between 1 MHz and 100 MHz to determine the acoustic radiation resulted from interactions of particles that experience nuclear spins as a result of magnetic and/or electric fields. The principles of nuclear acoustic resonance are often compared with nuclear magnetic resonance, specifically its usage in conjunction with nuclear magnetic resonance systems for spectroscopy and related imaging methodologies. Due to this, it is denoted that nuclear acoustic resonance can be used for the imaging of objects as well. However, for most cases, nuclear acoustic resonance requires the presence of nuclear magnetic resonance to induce electron spins within specimens in order for the absorption of acoustic waves to occur. Research conducted through experimental and theoretical investigations relative to the absorption of acoustic radiation of different materials, ranging from metals to subatomic particles, have deducted that nuclear acoustic resonance has its specific usages in other fields other than imaging. Experimental observations of nuclear acoustic resonance was first obtained in 1963 by Alers and Fleury in solid aluminum.












