Related Research Articles

Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.

Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Cavity ring-down spectroscopy (CRDS) is a highly sensitive optical spectroscopic technique that enables measurement of absolute optical extinction by samples that scatter and absorb light. It has been widely used to study gaseous samples which absorb light at specific wavelengths, and in turn to determine mole fractions down to the parts per trillion level. The technique is also known as cavity ring-down laser absorption spectroscopy (CRLAS).

A quartz crystal microbalance (QCM) measures a mass variation per unit area by measuring the change in frequency of a quartz crystal resonator. The resonance is disturbed by the addition or removal of a small mass due to oxide growth/decay or film deposition at the surface of the acoustic resonator. The QCM can be used under vacuum, in gas phase and more recently in liquid environments. It is useful for monitoring the rate of deposition in thin-film deposition systems under vacuum. In liquid, it is highly effective at determining the affinity of molecules to surfaces functionalized with recognition sites. Larger entities such as viruses or polymers are investigated as well. QCM has also been used to investigate interactions between biomolecules. Frequency measurements are easily made to high precision ; hence, it is easy to measure mass densities down to a level of below 1 μg/cm2. In addition to measuring the frequency, the dissipation factor is often measured to help analysis. The dissipation factor is the inverse quality factor of the resonance, Q−1 = w/fr ; it quantifies the damping in the system and is related to the sample's viscoelastic properties.

Resonance Raman spectroscopy is a variant of Raman spectroscopy in which the incident photon energy is close in energy to an electronic transition of a compound or material under examination. This similarity in energy (resonance) leads to greatly increased intensity of the Raman scattering of certain vibrational modes, compared to ordinary Raman spectroscopy.
Infrared multiple photon dissociation (IRMPD) is a technique used in mass spectrometry to fragment molecules in the gas phase usually for structural analysis of the original (parent) molecule.

Electron-capture dissociation (ECD) is a method of fragmenting gas-phase ions for structure elucidation of peptides and proteins in tandem mass spectrometry. It is one of the most widely used techniques for activation and dissociation of mass selected precursor ion in MS/MS. It involves the direct introduction of low-energy electrons to trapped gas-phase ions.

Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa), commonly coupled with high-performance liquid chromatography (HPLC). APCI is a soft ionization method similar to chemical ionization where primary ions are produced on a solvent spray. The main usage of APCI is for polar and relatively less polar thermally stable compounds with molecular weight less than 1500 Da. The application of APCI with HPLC has gained a large popularity in trace analysis detection such as steroids, pesticides and also in pharmacology for drug metabolites.
In a chemical analysis, the internal standard method involves adding the same amount of a chemical substance to each sample and calibration solution. The internal standard responds proportionally to changes in the analyte and provides a similar, but not identical, measurement signal. It must also be absent from the sample matrix to ensure there is no other source of the internal standard present. Taking the ratio of analyte signal to internal standard signal and plotting it against the analyte concentrations in the calibration solutions will result in a calibration curve. The calibration curve can then be used to calculate the analyte concentration in an unknown sample.

Acoustic levitation is a method for suspending matter in air against gravity using acoustic radiation pressure from high intensity sound waves.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.

Matrix-assisted laser desorption electrospray ionization (MALDESI) was first introduced in 2006 as a novel ambient ionization technique which combines the benefits of electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI). An infrared (IR) or ultraviolet (UV) laser can be utilized in MALDESI to resonantly excite an endogenous or exogenous matrix. The term 'matrix' refers to any molecule that is present in large excess and absorbs the energy of the laser, thus facilitating desorption of analyte molecules. The original MALDESI design was implemented using common organic matrices, similar to those used in MALDI, along with a UV laser. The current MALDESI source employs endogenous water or a thin layer of exogenously deposited ice as the energy-absorbing matrix where O-H symmetric and asymmetric stretching bonds are resonantly excited by a mid-IR laser.
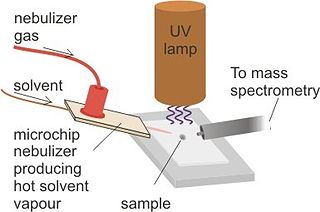
Desorption atmospheric pressure photoionization (DAPPI) is an ambient ionization technique for mass spectrometry that uses hot solvent vapor for desorption in conjunction with photoionization. Ambient Ionization techniques allow for direct analysis of samples without pretreatment. The direct analysis technique, such as DAPPI, eliminates the extraction steps seen in most nontraditional samples. DAPPI can be used to analyze bulkier samples, such as, tablets, powders, resins, plants, and tissues. The first step of this technique utilizes a jet of hot solvent vapor. The hot jet thermally desorbs the sample from a surface. The vaporized sample is then ionized by the vacuum ultraviolet light and consequently sampled into a mass spectrometer. DAPPI can detect a range of both polar and non-polar compounds, but is most sensitive when analyzing neutral or non-polar compounds. This technique also offers a selective and soft ionization for highly conjugated compounds.

Ambient ionization is a form of ionization in which ions are formed in an ion source outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by chemical ionization, or laser desorbed or ablated and post-ionized before they enter the mass spectrometer.
In statistics, the bootstrap error-adjusted single-sample technique is a non-parametric method that is intended to allow an assessment to be made of the validity of a single sample. It is based on estimating a probability distribution representing what can be expected from valid samples. This is done use a statistical method called bootstrapping, applied to previous samples that are known to be valid.

Atmospheric pressure photoionization (APPI) is a soft ionization method used in mass spectrometry (MS) usually coupled to liquid chromatography (LC). Molecules are ionized using a vacuum ultraviolet (VUV) light source operating at atmospheric pressure, either by direct absorption followed by electron ejection or through ionization of a dopant molecule that leads to chemical ionization of target molecules. The sample is usually a solvent spray that is vaporized by nebulization and heat. The benefit of APPI is that it ionizes molecules across a broad range of polarity and is particularly useful for ionization of low polarity molecules for which other popular ionization methods such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) are less suitable. It is also less prone to ion suppression and matrix effects compared to ESI and APCI and typically has a wide linear dynamic range. The application of APPI with LC/MS is commonly used for analysis of petroleum compounds, pesticides, steroids, and drug metabolites lacking polar functional groups and is being extensively deployed for ambient ionization particularly for explosives detection in security applications.

Resonance ionization is a process in optical physics used to excite a specific atom beyond its ionization potential to form an ion using a beam of photons irradiated from a pulsed laser light. In resonance ionization, the absorption or emission properties of the emitted photons are not considered, rather only the resulting excited ions are mass-selected, detected and measured. Depending on the laser light source used, one electron can be removed from each atom so that resonance ionization produces an efficient selectivity in two ways: elemental selectivity in ionization and isotopic selectivity in measurement.
Probe electrospray ionization (PESI) is an electrospray-based ambient ionization technique which is coupled with mass spectrometry for sample analysis. Unlike traditional mass spectrometry ion sources which must be maintained in a vacuum, ambient ionization techniques permit sample ionization under ambient conditions, allowing for the high-throughput analysis of samples in their native state, often with minimal or no sample pre-treatment. The PESI ion source simply consists of a needle to which a high voltage is applied following sample pick-up, initiating electrospray directly from the solid needle.
References
- 1 2 DiGregorio, Barry E. (2007). "AC Detective: All you need is sound". Analytical Chemistry. American Chemical Society (ACS). 79 (19): 7236. doi: 10.1021/ac071966x . ISSN 0003-2700.
- ↑ Lai, Edward P. C.; Chan, Becky L.; Chen, Susan (1988). "Ultrasonic Resonance Spectroscopic Analysis of Microliters of Liquids". Applied Spectroscopy. SAGE Publications. 42 (3): 526–529. Bibcode:1988ApSpe..42..526L. doi:10.1366/0003702884427906. ISSN 0003-7028. S2CID 94787680.
- 1 2 Buice, Robert G.; Pinkston, Paul; Lodder, Robert A. (1994). "Optimization of Acoustic-Resonance Spectrometry for Analysis of Intact Tablets and Prediction of Dissolution Rate". Applied Spectroscopy. SAGE Publications. 48 (4): 517–524. Bibcode:1994ApSpe..48..517B. doi:10.1366/000370294775268929. ISSN 0003-7028. S2CID 10416928.
- 1 2 Hannel, Thaddaeus; Link, David; Lodder, Robert A. (14 August 2008). "Integrated Sensing and Processing—Acoustic Resonance Spectrometry (ISP-ARS) in Differentiating d-Tagatose and Other Toll Manufactured Drugs". Journal of Pharmaceutical Innovation. Springer Science and Business Media LLC. 3 (3): 152–160. doi:10.1007/s12247-008-9038-y. ISSN 1872-5120. S2CID 177787.
- ↑ Medendorp, Joseph P.; Fackler, Jason A.; Douglas, Craig C.; Lodder, Robert A. (2007). "Integrated Sensing and Processing Acoustic Resonance Spectrometry (ISP-ARS) for Sample Classification". Journal of Pharmaceutical Innovation. Springer Science and Business Media LLC. 2 (3–4): 125–134. doi:10.1007/s12247-007-9014-y. ISSN 1872-5120. S2CID 6064202.
- ↑ Cutnell, J. D.; Johnson, K. W., Physics. Wiley: New York, 1997.
- ↑ Franco-Villafañe, J A; Flores-Olmedo, E; Báez, G; Gandarilla-Carrillo, O; Méndez-Sánchez, R A (3 October 2012). "Acoustic resonance spectroscopy for the advanced undergraduate laboratory". European Journal of Physics. IOP Publishing. 33 (6): 1761–1769. arXiv: 1312.5611 . Bibcode:2012EJPh...33.1761F. doi:10.1088/0143-0807/33/6/1761. ISSN 0143-0807. S2CID 54058402.
- ↑ Kourtiche, D; Ali, L Ait; Alliès, L; Nadi, M; Chitnalah, A (14 October 2003). "Harmonic propagation of finite-amplitude sound beams: second harmonic imaging in ultrasonic reflection tomography". Measurement Science and Technology. IOP Publishing. 15 (1): 21–28. doi:10.1088/0957-0233/15/1/003. ISSN 0957-0233. S2CID 250826581.
- ↑ Mills, Timothy P.; Jones, Angela; Lodder, Robert A. (1993). "Identification of Wood Species by Acoustic-Resonance Spectrometry Using Multivariate Subpopulation Analysis". Applied Spectroscopy. SAGE Publications. 47 (11): 1880–1886. Bibcode:1993ApSpe..47.1880M. doi:10.1366/0003702934065957. ISSN 0003-7028. S2CID 17775719.
- ↑ Mills, T.; Nair, P.; Chandrasekaran, S.; Lodder, R. "Improved identification of pharmaceutical tablets by near-IR and near-IR / acoustic-resonance spectrometry with bootstrap principal components".
- ↑ Soil Sci. Soc. Am. J., Vol. 68, January–February 2004
- ↑ Martin, L.P.; Poret, J.C.; Danon, A.; Rosen, M. (1998). "Effect of adsorbed water on the ultrasonic velocity in alumina powder compacts". Materials Science and Engineering: A. Elsevier BV. 252 (1): 27–35. doi: 10.1016/s0921-5093(98)00669-8 . ISSN 0921-5093.
- ↑ Medendorp, Joseph; Lodder, Robert A. (2006). "Acoustic-resonance spectrometry as a process analytical technology for rapid and accurate tablet identification". AAPS PharmSciTech. Springer Science and Business Media LLC. 7 (1): E175–E183. doi:10.1208/pt070125. ISSN 1530-9932. PMC 2750732 . PMID 16584156.
- ↑ Medendorp, Joseph; Buice, Robert G.; Lodder, Robert A. (2006). "Acoustic-resonance spectrometry as a process analytical technology for the quantification of active pharmaceutical ingredient in semi-solids". AAPS PharmSciTech. Springer Science and Business Media LLC. 7 (3): E22–E29. doi:10.1208/pt070359. ISSN 1530-9932. PMC 2750501 . PMID 16584153.
- Zhang, Rui; Jiang, Bei; Cao, Wenwu (2002). "Influence of sample size on ultrasonic phase velocity measurements in piezoelectric ceramics". Journal of Applied Physics. AIP Publishing. 91 (12): 10194. Bibcode:2002JAP....9110194Z. doi:10.1063/1.1479754. ISSN 0021-8979.
- Liu, Qiong; Lange, Rebecca A.; Ai, Yuhui (2007). "Acoustic velocity measurements on Na2O–TiO2–SiO2 liquids: Evidence for a highly compressible TiO2 component related to five-coordinated Ti". Geochimica et Cosmochimica Acta. Elsevier BV. 71 (17): 4314–4326. Bibcode:2007GeCoA..71.4314L. doi:10.1016/j.gca.2007.06.054. ISSN 0016-7037.
- Baldwin, Steven L.; Marutyan, Karen R.; Yang, Min; Wallace, Kirk D.; Holland, Mark R.; Miller, James G. (2006). "Measurements of the anisotropy of ultrasonic attenuation in freshly excised myocardium". The Journal of the Acoustical Society of America. Acoustical Society of America (ASA). 119 (5): 3130–3139. Bibcode:2006ASAJ..119.3130B. doi: 10.1121/1.2188333 . ISSN 0001-4966. PMID 16708967.
- Umnova, Olga; Attenborough, Keith; Shin, Ho-Chul; Cummings, Alan (2005). "Deduction of tortuosity and porosity from acoustic reflection and transmission measurements on thick samples of rigid-porous materials". Applied Acoustics. Elsevier BV. 66 (6): 607–624. doi:10.1016/j.apacoust.2004.02.005. ISSN 0003-682X.
- Lei, Xinglin; Masuda, Koji; Nishizawa, Osamu; Jouniaux, Laurence; Liu, Liqiang; Ma, Wentao; Satoh, Takashi; Kusunose, Kinichiro (2004). "Detailed analysis of acoustic emission activity during catastrophic fracture of faults in rock". Journal of Structural Geology. Elsevier BV. 26 (2): 247–258. Bibcode:2004JSG....26..247L. doi:10.1016/s0191-8141(03)00095-6. ISSN 0191-8141.
- Kunkler-Peck, Andrew J.; Turvey, M. T. (2000). "Hearing shape". Journal of Experimental Psychology: Human Perception and Performance. American Psychological Association (APA). 26 (1): 279–294. doi:10.1037/0096-1523.26.1.279. ISSN 1939-1277. PMID 10696618.
- Gordon, Michael S.; Rosenblum, Lawrence D. (2004). "Perception of Sound-Obstructing Surfaces Using Body-Scaled Judgments". Ecological Psychology. Informa UK Limited. 16 (2): 87–113. doi:10.1207/s15326969eco1602_1. ISSN 1040-7413. S2CID 144740329.
- Sinha, D.N. (1992). "Acoustic resonance spectroscopy (ARS)". IEEE Potentials. Institute of Electrical and Electronics Engineers (IEEE). 11 (2): 10–13. doi:10.1109/45.127718. ISSN 0278-6648. S2CID 42159817.