
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group.

Phenolphthalein ( feh-NOL(F)-thə-leen) is a chemical compound with the formula C20H14O4 and is often written as "HIn", "HPh", "phph" or simply "Ph" in shorthand notation. Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colorless in acidic solutions and pink in basic solutions. It belongs to the class of dyes known as phthalein dyes.
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.

Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms. The term "unsaturated" means more hydrogen atoms may be added to the hydrocarbon to make it saturated. The configuration of an unsaturated carbons include straight chain, such as alkenes and alkynes, as well as branched chains and aromatic compounds.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
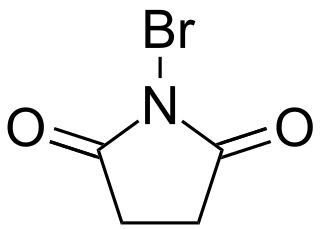
N-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical.

Maleic acid or cis-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer. It is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, maleic acid has few applications.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.

A halonium ion is any onium ion containing a halogen atom carrying a positive charge. This cation has the general structure R−+X−R′ where X is any halogen and no restrictions on R, this structure can be cyclic or an open chain molecular structure. Halonium ions formed from fluorine, chlorine, bromine, and iodine are called fluoronium, chloronium, bromonium, and iodonium, respectively. The 3-membered cyclic variety commonly proposed as intermediates in electrophilic halogenation may be called haliranium ions, using the Hantzsch-Widman nomenclature system.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
In organic chemistry, the bromine test is a qualitative test for the presence of unsaturation, phenols and anilines.
Organobromine compounds, also called organobromides, are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
2-Bromobutane is an isomer of 1-bromobutane. Both compounds share the molecular formula C4H9Br. 2-Bromobutane is also known as sec-butyl bromide or methylethylbromomethane. Because it contains bromine, a halogen, it is part of a larger class of compounds known as alkyl halides. It is a colorless liquid with a pleasant odor. Because the carbon atom connected to the bromine is connected to two other carbons the molecule is referred to as a secondary alkyl halide. 2-Bromobutane is chiral and thus can be obtained as either of two enantiomers designated as (R)-(−)-2-bromobutane and (S)-(+)-2-bromobutane.

Methylcyclohexene refers to any one of three organic compounds consisting of cyclohexene with a methyl group substituent. The location of the methyl group relative to the cyclohexene double bond creates the three different structural isomers. These compounds are generally used as a reagent or intermediate to derive other organic compounds.
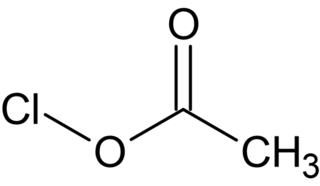
Acetyl hypochlorite, also known as chlorine acetate, is a chemical compound with the formula CH3COOCl. It is a photosensitive colorless liquid that is a short lived intermediate in the Hunsdiecker reaction.










