
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a "deliquescent crystal or wax-like solid with a pungent, sweet, corn-like, nutty odour".

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.
The Combes quinoline synthesis is a chemical reaction, which was first reported by Combes in 1888. Further studies and reviews of the Combes quinoline synthesis and its variations have been published by Alyamkina et al., Bergstrom and Franklin, Born, and Johnson and Mathews.

Oxamide is the organic compound with the formula (CONH2)2. This white crystalline solid is soluble in ethanol, slightly soluble in water and insoluble in diethyl ether. Oxamide is the diamide derived from oxalic acid, and the hydrate of cyanogen.
ChemSpider is a freely accessible online database of chemicals owned by the Royal Society of Chemistry. It contains information on more than 100 million molecules from over 270 data sources, each of them receiving a unique identifier called ChemSpider Identifier.

Prenalterol is a cardiac stimulant which acts as a β1 adrenoreceptor agonist.

Dioxaphetyl butyrate is an opioid analgesic which is a diphenylacetic acid derivative, related to other open-chain opioid drugs such as dextropropoxyphene, levacetylmethadol (LAAM), lefetamine and dimenoxadol.

N-n-Propylnorapomorphine (NPA) is an aporphine derivative dopamine agonist closely related to apomorphine. In rodents it has been shown to produce hyperactivity, stereotypy, hypothermia, antinociception, and penile erection, among other effects. Notably, its effects on locomotion are biphasic, with low doses producing inhibition and catalepsy and high doses resulting in enhancement of activity. This is likely due to preferential activation of D2/D3 autoreceptors versus postsynaptic receptors, the latter of which overcomes the former to increase postsynaptic dopaminergic signaling only with high doses.

Dacemazine (INN, also known as Ahistan and Histantine) is a phenothiazine derivative which acts as a histamine antagonist at the H1 subtype. First described in 1951, it was never marketed as a drug on its own, although a combination of dacemazine and di-tert-butylnaphthalenesulfonate was sold as an antispasmodic and antitussive under the trade name Codopectyl. It was also assessed as a possible anticancer drug.
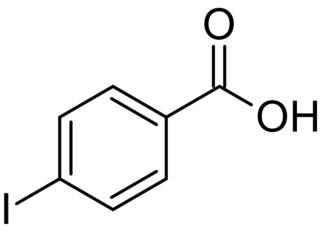
Iodobenzoic acids are any of three organic compounds with the formula IC6H4COOH, consisting of a carboxylic acid group and an iodine atom bonded to a central benzene ring. They can be considered as iodinated derivatives of benzoic acid, or as carboxylated variants of iodobenzene.

Desocodeine is a potent semi-synthetic opioid which is the penultimate intermediate in the manufacture of desomorphine from codeine. Desocodeine is a potent analgesic, being as potent as morphine.It is partially metabolized into desomorphine, among others, after parenteral and oral administration.

7α-Thioprogesterone is a synthetic, steroidal, and potent antimineralocorticoid (putative) and antiandrogen which was developed by G. D. Searle & Co and was described in the late 1970s and early 1980s but was never developed or introduced for medical use. It is a derivative of progesterone (pregn-4-ene-3,20-dione) with a thio (sulfur) substitution at the C7α position, and is related to the spirolactone group of drugs but lacks a γ-lactone ring.

Bromethenmadinone acetate is a progestin medication which was developed in Czechoslovakia and was described in 1970 but was never marketed. Analogues of BMMA include chlormethenmadinone acetate, melengestrol acetate, and methenmadinone acetate.
Diaminonaphthalene describes several isomers containing naphthalene substituted with two amine groups (NH2), also called naphthalenediamines. All isomers are white solids that tend to air-oxidize. The 2,3-, 1,5-, and 1,8- derivatives have attracted most attention.
1,6-Hexanediol diacrylate is a difunctional acrylate ester monomer used in the manufacture of polymers. It is particularly useful for use in ultraviolet light cure applications. Furthermore, it is also used in adhesives, sealants, alkyd coatings, elastomers, photopolymers, and inks for improved adhesion, hardness, abrasion and heat resistance. Like other acrylate monomers it is usually supplied with a radical inhibitor such as hydroquinone added.
Bromotoluenes are aryl bromides based on toluene in which at least one aromatic hydrogen atom is replaced with a bromine atom. They have the general formula C7H8–nBrn, where n = 1–5 is the number of bromine atoms.

4-Bromobenzaldehyde, or p-bromobenzaldehyde, is an isomer of bromobenzaldehyde.

4-Ethynylbenzaldehyde, or p-ethynylbenzaldehyde, is an organic compound with the formula HC2C6H4COH. It is an ethynyl derivative of benzaldehyde, or may also be viewed as a formylated derivative of phenylacetylene.















