Related Research Articles
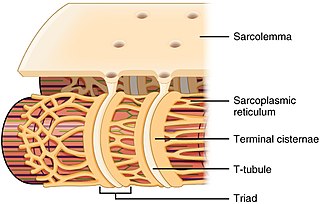
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.
SERCA, or sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, or SR Ca2+-ATPase, is a calcium ATPase-type P-ATPase. Its major function is to transport calcium from the cytosol into the sarcoplasmic reticulum.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.

Phospholamban, also known as PLN or PLB, is a micropeptide protein that in humans is encoded by the PLN gene. Phospholamban is a 52-amino acid integral membrane protein that regulates the calcium (Ca2+) pump in cardiac muscle cells.

T-tubules are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. With membranes that contain large concentrations of ion channels, transporters, and pumps, T-tubules permit rapid transmission of the action potential into the cell, and also play an important role in regulating cellular calcium concentration.

Amrinone, also known as inamrinone, and sold as Inocor, is a pyridine phosphodiesterase 3 inhibitor. It is a drug that may improve the prognosis in patients with congestive heart failure. Amrinone has been shown to increase the contractions initiated in the heart by high-gain calcium induced calcium release (CICR). The positive inotropic effect of amrinone is mediated by the selective enhancement of high-gain CICR, which contributes to the contraction of myocytes by phosphorylation through cAMP dependent protein kinase A (PKA) and Ca2+ calmodulin kinase pathways.

Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited genetic disorder that predisposes those affected to potentially life-threatening abnormal heart rhythms or arrhythmias. The arrhythmias seen in CPVT typically occur during exercise or at times of emotional stress, and classically take the form of bidirectional ventricular tachycardia or ventricular fibrillation. Those affected may be asymptomatic, but they may also experience blackouts or even sudden cardiac death.
A calcium spark is the microscopic release of calcium (Ca2+) from a store known as the sarcoplasmic reticulum (SR), located within muscle cells. This release occurs through an ion channel within the membrane of the SR, known as a ryanodine receptor (RyR), which opens upon activation. This process is important as it helps to maintain Ca2+ concentration within the cell. It also initiates muscle contraction in skeletal and cardiac muscles and muscle relaxation in smooth muscles. Ca2+ sparks are important in physiology as they show how Ca2+ can be used at a subcellular level, to signal both local changes, known as local control, as well as whole cell changes.
The Anrep effect is an autoregulation method in which myocardial contractility increases with afterload. It was experimentally determined that increasing afterload caused a proportional linear increase in ventricular inotropy. This effect is found in denervated heart preparations, such as the Starling Preparation, and represents an intrinsic autoregulation mechanism.
Afterdepolarizations are abnormal depolarizations of cardiac myocytes that interrupt phase 2, phase 3, or phase 4 of the cardiac action potential in the electrical conduction system of the heart. Afterdepolarizations may lead to cardiac arrhythmias. Afterdepolarization is commonly a consequence of myocardial infarction, cardiac hypertrophy, or heart failure. It may also result from congenital mutations associated with calcium channels and sequestration.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.
Imperatoxin I (IpTx) is a peptide toxin derived from the venom of the African scorpion Pandinus imperator.
JTV-519 (K201) is a 1,4-benzothiazepine derivative that interacts with many cellular targets. It has many structural similarities to diltiazem, a Ca2+ channel blocker used for treatment of hypertension, angina pectoris and some types of arrhythmias. JTV-519 acts in the sarcoplasmic reticulum (SR) of cardiac myocytes by binding to and stabilizing the ryanodine receptor (RyR2) in its closed state. It can be used in the treatment of cardiac arrhythmias, heart failure, catecholaminergic polymorphic ventricular tachycardia (CPVT) and store overload-induced Ca2+ release (SOICR). Currently, this drug has only been tested on animals and its side effects are still unknown. As research continues, some studies have also found a dose-dependent response; where there is no improvement seen in failing hearts at 0.3 μM and a decline in response at 1 μM.

Istaroxime is an investigational drug under development for treatment of acute decompensated heart failure
Calcium buffering describes the processes which help stabilise the concentration of free calcium ions within cells, in a similar manner to how pH buffers maintain a stable concentration of hydrogen ions. The majority of calcium ions within the cell are bound to intracellular proteins, leaving a minority freely dissociated. When calcium is added to or removed from the cytoplasm by transport across the cell membrane or sarcoplasmic reticulum, calcium buffers minimise the effect on changes in cytoplasmic free calcium concentration by binding calcium to or releasing calcium from intracellular proteins. As a result, 99% of the calcium added to the cytosol of a cardiomyocyte during each cardiac cycle becomes bound to calcium buffers, creating a relatively small change in free calcium.

Hadrucalcin is a peptide toxin from the venom of the scorpion Hadrurus gertschi. Hadrucalcin modifies the Ryanodine receptor channels RyR1 and RyR2, found in the sarcoplasmic reticulum, to a long-lasting subconductance state, thus inducing the release of calcium from the sarcoplasmic reticulum.
The dyadic space is the name for the volume of cytoplasm between pairs (dyads) of areas where the cell membrane and an organelle such as the endoplasmic reticulum come into close contact of each other, creating what are known as dyadic clefts.
The ryanodine-inositol 1,4,5-triphosphate receptor Ca2+ channel (RIR-CaC) family includes Ryanodine receptors and Inositol trisphosphate receptors. Members of this family are large proteins, some exceeding 5000 amino acyl residues in length. This family belongs to the Voltage-gated ion channel (VIC) superfamily. Ry receptors occur primarily in muscle cell sarcoplasmic reticular (SR) membranes, and IP3 receptors occur primarily in brain cell endoplasmic reticular (ER) membranes where they effect release of Ca2+ into the cytoplasm upon activation (opening) of the channel. They are redox sensors, possibly providing a partial explanation for how they control cytoplasmic Ca2+. Ry receptors have been identified in heart mitochondria where they provide the main pathway for Ca2+ entry. Sun et al. (2011) have demonstrated oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel (RyR1;TC# 1.A.3.1.2) by NADPH oxidase 4.
Cardiac excitation-contraction coupling (CardiacEC coupling) describes the series of events, from the production of an electrical impulse (action potential) to the contraction of muscles in the heart. This process is of vital importance as it allows for the heart to beat in a controlled manner, without the need for conscious input. EC coupling results in the sequential contraction of the heart muscles that allows blood to be pumped, first to the lungs (pulmonary circulation) and then around the rest of the body (systemic circulation) at a rate between 60 and 100 beats every minute, when the body is at rest. This rate can be altered, however, by nerves that work to either increase heart rate (sympathetic nerves) or decrease it (parasympathetic nerves), as the body's oxygen demands change. Ultimately, muscle contraction revolves around a charged atom (ion), calcium (Ca2+), which is responsible for converting the electrical energy of the action potential into mechanical energy (contraction) of the muscle. This is achieved in a region of the muscle cell, called the transverse tubule during a process known as calcium induced calcium release.
References
- ↑ Del Rio, Carlos; Yukie Ueyama; Robert L. Hamlin; John Reardon; Reza Mazhari (April 2011). "CXL-1020, A Novel Hno Donor, Decreases Myocardial Loading and Enhances Load-Independent Lusitropy and Inotropy Via A ?-Ar/Ace Independent Mechanism". JACC. 57 (17): E326. doi: 10.1016/s0735-1097(11)60326-4 .
- 1 2 3 Kohr, Mark; Nina Kaludercic; Carlo Tocchetti; Wei Dong Gao; David Kass; Paul Janssen; Nazareno Paolocci; Mark Ziolo (March 2011). "Nitroxyl enhances myocyte Ca2+ transients by exclusively targeting SR Ca2+-cycling". Frontiers in Bioscience. E2 (2): 614–626. doi:10.2741/e118. PMC 3057191 . PMID 20036906.
- 1 2 Dai, Tieying; Ye Tian; Carlo Tocchetti; Tatsuo Katori; Anne Murphy; David Kass; Nazareno Paolocci; Wei Dong Gao (2007). "Nitroxyl increases force development in rat cardiac muscle". Journal of Physiology. 580 (3): 951–960. doi:10.1113/jphysiol.2007.129254. PMC 2075441 . PMID 17331988.
- ↑ Wang, Mengjun; Reza Mazhari; Itamar Ilsar; Alice Wang; Michael Sabbah; Hani Sabbah (2009). "Intravenous Infusion of CXL-1020, a Novel Nitroxyl (HNO) Donor, Improves Left Ventricular Systolic and Diastolic Function in Dogs with Advanced Heart Failure". Circulation. 120: S582.
- ↑ Lancel, Steve; Jingmei Zhang; Alicia Evangelista; Mario Trucillo; XiaoYong Tong; Deborah Siwik; Richard Cohen; Wilson Colucci (2009). "Nitroxyl Activates SERCA in Cardiac Myocytes via Glutathiolation of Cysteine 674". Circulation Research. 104 (6): 720–723. doi:10.1161/circresaha.108.188441. PMC 3046805 . PMID 19265039.
- 1 2 Tocchetti, Carlo; Wang Wang; Jeffrey Froehlich; Sabine Huke; Miguel Aon; Gerald Wilson; Giulietta Di Benedetto; Brian O'Rourke; Wei Dong Gao; David Wink; John Toscano; Manuela Zaccolo; Donald Bers; Hector Valdivia; Heping Cheng; David Kass; Nazareno Paolocci (2007). "Nitroxyl Improves Cellular Heart Function by Directly Enhancing Cardiac Sarcoplasmic Reticulum Ca 2+ Cycling". Circulation Research. 100 (1): 96–104. doi:10.1161/01.res.0000253904.53601.c9. PMC 2769513 . PMID 17138943.