
Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.
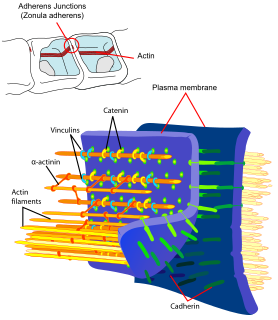
Cadherins (named for "calcium-dependent adhesion") are a type of cell adhesion molecule (CAM) that is important in the formation of adherens junctions to bind cells with each other. Cadherins are a class of type-1 transmembrane proteins. They are dependent on calcium (Ca2+) ions to function, hence their name. Cell-cell adhesion is mediated by extracellular cadherin domains, whereas the intracellular cytoplasmic tail associates with numerous adaptor and signaling proteins, collectively referred to as the cadherin adhesome.
Cell adhesion molecules (CAMs) are a subset of cell adhesion proteins located on the cell surface involved in binding with other cells or with the extracellular matrix (ECM) in the process called cell adhesion. In essence, cell adhesion molecules help cells stick to each other and to their surroundings. Cell adhesion is a crucial component in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in creating force and movement and consequently ensure that organs are able to execute their functions. In addition to serving as "molecular glue", cell adhesion is important in affecting cellular mechanisms of growth, contact inhibition, and apoptosis. Oftentimes aberrant expression of CAMs will result in pathologies ranging from frostbite to cancer.
Annexin is a common name for a group of cellular proteins. They are mostly found in eukaryotic organisms.

Protocadherins (Pcdhs) are the largest mammalian subgroup of the cadherin superfamily of homophilic cell-adhesion proteins. They were discovered by Shintaro Suzuki's group, when they used PCR to find new members of the cadherin family. The PCR fragments that corresponded to Protocadherins were found in vertebrate and invertebrate species. This prevalence in a wide range of species suggested that the fragments were part of an ancient cadherin and were thus termed "Protocadherins" as the "first cadherins". Of the approximately 70 Pcdh genes identified in mammalian genomes, over 50 are located in tightly linked gene clusters on the same chromosome. Until recently, it was assumed that this kind of organization can only be found in vertebrates, but Octopus bimaculoides has 168 genes of which nearly three-quarters are found in tandem clusters with the two largest clusters compromising 31 and 17 genes, respectively.
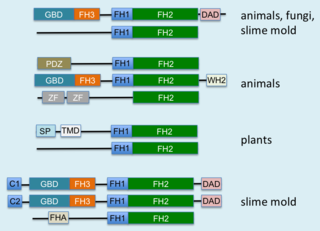
Formins are a group of proteins that are involved in the polymerization of actin and associate with the fast-growing end of actin filaments. Most formins are Rho-GTPase effector proteins. Formins regulate the actin and microtubule cytoskeleton and are involved in various cellular functions such as cell polarity, cytokinesis, cell migration and SRF transcriptional activity. Formins are multidomain proteins that interact with diverse signalling molecules and cytoskeletal proteins, although some formins have been assigned functions within the nucleus.
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions.

Cadherin 5, type 2 or VE-cadherin also known as CD144, is a type of cadherin. It is encoded by the human gene CDH5.

Catenin delta-1 is a protein that in humans is encoded by the CTNND1 gene.

Receptor-type tyrosine-protein phosphatase mu is an enzyme that in humans is encoded by the PTPRM gene.

Cadherin-4 is a protein that in humans is encoded by the CDH4 gene.

Receptor-type tyrosine-protein phosphatase T is an enzyme that in humans is encoded by the PTPRT gene.

Cadherin-1 also known as CAM 120/80 or epithelial cadherin (E-cadherin) or uvomorulin is a protein that in humans is encoded by the CDH1 gene. CDH1 has also been designated as CD324. It is a tumor suppressor gene.

In molecular biology, Phosphotyrosine-binding domains are protein domains which bind to phosphotyrosine.

In molecular biology, chaperone DnaJ, also known as Hsp40, is a molecular chaperone protein. It is expressed in a wide variety of organisms from bacteria to humans.
WH1 domain is an evolutionary conserved protein domain. Therefore, it has an important function.
In molecular biology, the FERM domain is a widespread protein module involved in localising proteins to the plasma membrane. FERM domains are found in a number of cytoskeletal-associated proteins that associate with various proteins at the interface between the plasma membrane and the cytoskeleton. The FERM domain is located at the N terminus in the majority of proteins in which it is found.
Long-term potentiation (LTP), thought to be the cellular basis for learning and memory, involves a specific signal transmission process that underlies synaptic plasticity. Among the many mechanisms responsible for the maintenance of synaptic plasticity is the cadherin–catenin complex. By forming complexes with intracellular catenin proteins, neural cadherins (N-cadherins) serve as a link between synaptic activity and synaptic plasticity, and play important roles in the processes of learning and memory.

The E3 ubiquitin-protein ligase Hakai (HAKAI) also known as Casitas B-lineage lymphoma-transforming sequence-like protein 1 (CBLL1) is an enzyme that in humans is encoded by the CBLL1 gene. This gene encodes an E3 ubiquitin ligase for the E-cadherin complex and mediates its ubiquitination, endocytosis, and degradation in the lysosomes. The encoded protein contains a RING-finger domain and is also thought to have a role in control of cell proliferation.

Synaptic stabilization is crucial in the developing and adult nervous systems and is considered a result of the late phase of long-term potentiation (LTP). The mechanism involves strengthening and maintaining active synapses through increased expression of cytoskeletal and extracellular matrix elements and postsynaptic scaffold proteins, while pruning less active ones. For example, cell adhesion molecules (CAMs) play a large role in synaptic maintenance and stabilization. Gerald Edelman discovered CAMs and studied their function during development, which showed CAMs are required for cell migration and the formation of the entire nervous system. In the adult nervous system, CAMs play an integral role in synaptic plasticity relating to learning and memory.

















