Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
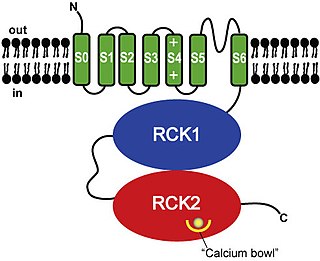
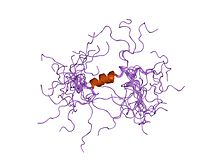
BK channels (big potassium), are large conductance calcium-activated potassium channels, also known as Maxi-K, slo1, or Kca1.1. BK channels are voltage-gated potassium channels that conduct large amounts of potassium ions (K+) across the cell membrane, hence their name, big potassium. These channels can be activated (opened) by either electrical means, or by increasing Ca2+ concentrations in the cell. BK channels help regulate physiological processes, such as circadian behavioral rhythms and neuronal excitability. BK channels are also involved in many processes in the body, as it is a ubiquitous channel. They have a tetrameric structure that is composed of a transmembrane domain, voltage sensing domain, potassium channel domain, and a cytoplasmic C-terminal domain, with many X-ray structures for reference. Their function is to repolarize the membrane potential by allowing for potassium to flow outward, in response to a depolarization or increase in calcium levels.
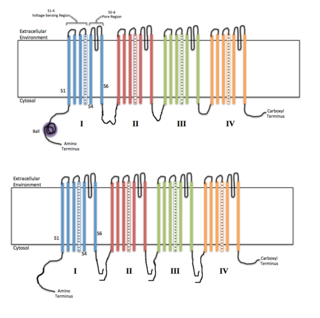
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.

Cyclic nucleotide–gated ion channels or CNG channels are ion channels that function in response to the binding of cyclic nucleotides. CNG channels are nonselective cation channels that are found in the membranes of various tissue and cell types, and are significant in sensory transduction as well as cellular development. Their function can be the result of a combination of the binding of cyclic nucleotides and either a depolarization or a hyperpolarization event. Initially discovered in the cells that make up the retina of the eye, CNG channels have been found in many different cell types across both the animal and the plant kingdoms. CNG channels have a very complex structure with various subunits and domains that play a critical role in their function. CNG channels are significant in the function of various sensory pathways including vision and olfaction, as well as in other key cellular functions such as hormone release and chemotaxis. CNG channels have also been found to exist in prokaryotes, including many spirochaeta, though their precise role in bacterial physiology remains unknown.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g., muscle, glial cells, neurons, etc.) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+-Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's plasma membrane. They belong to the superfamily of cation channels and can be classified according to the trigger that opens the channel for such ions, i.e. either a voltage-change ("voltage-gated", "voltage-sensitive", or "voltage-dependent" sodium channel; also called "VGSCs" or "Nav channel") or a binding of a substance (a ligand) to the channel (ligand-gated sodium channels).
Calcium-activated potassium channels are potassium channels gated by calcium, or that are structurally or phylogenetically related to calcium gated channels. They were first discovered in 1958 by Gardos who saw that Calcium levels inside of a cell could affect the permeability of potassium through that cell membrane. Then in 1970, Meech was the first to observe that intracellular calcium could trigger potassium currents. In humans they are divided into three subtypes: large conductance or BK channels, which have very high conductance which range from 100 to 300 pS, intermediate conductance or IK channels, with intermediate conductance ranging from 25 to 100 pS, and small conductance or SK channels with small conductances from 2-25 pS.

Voltage-gated potassium channels (VGKCs) are transmembrane channels specific for potassium and sensitive to voltage changes in the cell's membrane potential. During action potentials, they play a crucial role in returning the depolarized cell to a resting state.
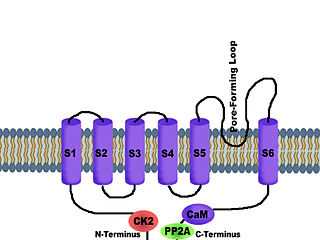
SK channels (small conductance calcium-activated potassium channels) are a subfamily of Ca2+-activated K+ channels. They are so called because of their small single channel conductance in the order of 10 pS. SK channels are a type of ion channel allowing potassium cations to cross the cell membrane and are activated (opened) by an increase in the concentration of intracellular calcium through N-type calcium channels. Their activation limits the firing frequency of action potentials and is important for regulating afterhyperpolarization in the neurons of the central nervous system as well as many other types of electrically excitable cells. This is accomplished through the hyperpolarizing leak of positively charged potassium ions along their concentration gradient into the extracellular space. This hyperpolarization causes the membrane potential to become more negative. SK channels are thought to be involved in synaptic plasticity and therefore play important roles in learning and memory.
T-type calcium channels are low voltage activated calcium channels that become deinactivated during cell membrane hyperpolarization but then open to depolarization. The entry of calcium into various cells has many different physiological responses associated with it. Within cardiac muscle cell and smooth muscle cells voltage-gated calcium channel activation initiates contraction directly by allowing the cytosolic concentration to increase. Not only are T-type calcium channels known to be present within cardiac and smooth muscle, but they also are present in many neuronal cells within the central nervous system. Different experimental studies within the 1970s allowed for the distinction of T-type calcium channels from the already well-known L-type calcium channels. The new T-type channels were much different from the L-type calcium channels due to their ability to be activated by more negative membrane potentials, had small single channel conductance, and also were unresponsive to calcium antagonist drugs that were present. These distinct calcium channels are generally located within the brain, peripheral nervous system, heart, smooth muscle, bone, and endocrine system.

The L-type calcium channel is part of the high-voltage activated family of voltage-dependent calcium channel. "L" stands for long-lasting referring to the length of activation. This channel has four subunits.

K+ channel tetramerisation domain is the N-terminal, cytoplasmic tetramerisation domain (T1) of voltage-gated K+ channels. It defines molecular determinants for subfamily-specific assembly of alpha-subunits into functional tetrameric channels. It is distantly related to the BTB/POZ domain Pfam PF00651.

Calcium-activated potassium channel subunit alpha-1 also known as large conductance calcium-activated potassium channel, subfamily M, alpha member 1 (KCa1.1), or BK channel alpha subunit, is a voltage gated potassium channel encoded by the KCNMA1 gene and characterized by their large conductance of potassium ions (K+) through cell membranes.

Calcium-activated potassium channel subunit beta-1 is a protein that in humans is encoded by the KCNMB1 gene.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.

Calcium channel, voltage-dependent, L type, alpha 1D subunit is a protein that in humans is encoded by the CACNA1D gene. Cav1.3 channels belong to the Cav1 family, which form L-type calcium currents and are sensitive to selective inhibition by dihydropyridines (DHP).
Calcium-activated potassium channel subunit beta-2 is a protein that in humans is encoded by the KCNMB2 gene.

Calcium-activated potassium channel subunit beta-3 is a protein that in humans is encoded by the KCNMB3 gene.

Calcium-activated potassium channel subunit beta-4 is a protein that in humans is encoded by the KCNMB4 gene.

Isopimaric acid (IPA) is a toxin which acts as a large conductance Ca2+-activated K+ channel (BK channel) opener.