The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory, by bombarding curium with alpha particles. It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye. The element was named after the university and the U.S. state of California.

Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.

Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic quantities, although pure fermium metal has not yet been prepared. A total of 19 isotopes are known, with 257Fm being the longest-lived with a half-life of 100.5 days.

Neutron activation analysis (NAA) is the nuclear process used for determining the concentrations of elements in many materials. NAA allows discrete sampling of elements as it disregards the chemical form of a sample, and focuses solely on atomic nuclei. The method is based on neutron activation and thus requires a source of neutrons. The sample is bombarded with neutrons, causing its constituent elements to form radioactive isotopes. The radioactive emissions and radioactive decay paths for each element have long been studied and determined. Using this information, it is possible to study spectra of the emissions of the radioactive sample, and determine the concentrations of the various elements within it. A particular advantage of this technique is that it does not destroy the sample, and thus has been used for the analysis of works of art and historical artifacts. NAA can also be used to determine the activity of a radioactive sample.

Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.
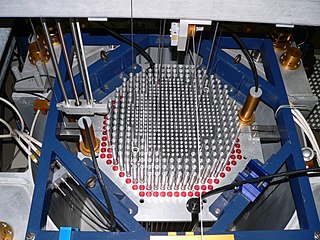
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid, which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. As of 2022, the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world.

Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear weapons reprocessing. The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and the environment.

A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear power.

In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". The typical radioisotope does not decay directly to a stable state, but rather it decays to another radioisotope. Thus there is usually a series of decays until the atom has become a stable isotope, meaning that the nucleus of the atom has reached a stable state.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.
Polonium-210 is an isotope of polonium. It undergoes alpha decay to stable 206Pb with a half-life of 138.376 days, the longest half-life of all naturally occurring polonium isotopes. First identified in 1898, and also marking the discovery of the element polonium, 210Po is generated in the decay chain of uranium-238 and radium-226. 210Po is a prominent contaminant in the environment, mostly affecting seafood and tobacco. Its extreme toxicity is attributed to intense radioactivity, capable of severely harming humans.

Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically.
Uranium-233 is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a reactor fuel. It has been used successfully in experimental nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years, plutonium-242 with a half-life of 373,300 years, and plutonium-239 with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states; all have half-lives of less than one second.
Californium (98Cf) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 245Cf in 1950. There are 20 known radioisotopes ranging from 237Cf to 256Cf and one nuclear isomer, 249mCf. The longest-lived isotope is 251Cf with a half-life of 898 years.
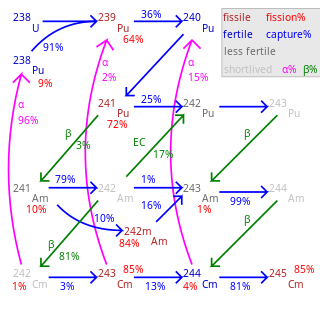
A minor actinide is an actinide found in spent nuclear fuel other than uranium or plutonium. The minor actinides include neptunium, americium, curium, berkelium, californium, einsteinium, and fermium. The most important isotopes of these elements in spent nuclear fuel are neptunium-237, americium-241, americium-243, curium-242 through -248, and californium-249 through -252.

The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwell distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The long wavelength of slow neutrons allows for the large cross section.











