Response properties
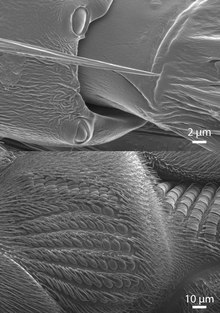
When cuticular deformations compress a campaniform sensillum, the socket edges (collar) indent the cuticular cap. [12] This squeezes the dendritic tip of the sensory neuron and opens its mechanotransduction channels (from the TRP family [13] ), which leads to the generation of action potentials that are transmitted to the ventral nerve cord, the insect analogue to the vertebrate spinal cord.
The activity of campaniform sensilla was first recorded by John William Sutton Pringle in the late 1930s. [14] Pringle also determined that the oval shape of many sensilla makes them directionally selective [15] – they respond best to compression along their short axis. Thus, even neighboring sensilla may have very different sensitivities to strain depending on their orientation in the cuticle. For example, stick insects possess two groups of campaniform sensilla on the dorsal side of their legs' trochanter whose short axes are oriented perpendicularly to one another [1] (see inset in leg schematic). As a result, one group (G3) responds when the leg is bent upwards, whereas the other group (G4) responds when the leg is bent downwards. Round campaniform sensilla can be sensitive in all directions [16] or show directional sensitivity if the cap is asymmetrically coupled with the surrounding collar. [17]
The activity of campaniform sensilla may be slowly-adapting (tonic), signaling the magnitude of cuticular deformation, and/or rapidly adapting (phasic), signaling the rate of cuticular deformation. [1] [18] Based on their responses to white noise stimuli, campaniform sensilla may also be described more generally as signaling two features that approximate the derivative of each other. [19] This suggests that the neural response properties of the sensilla are rather generic, and that functional specialization arises primarily from how the sensilla are embedded in the cuticle. [19] [20] In addition, activity adapts to constant loads and shows hysteresis (history dependence) in response to cyclic loading. [18]
Campaniform sensilla project directly to motor neurons [21] and to various interneurons, which integrate their signals with signals from other proprioceptors. [22] In this way, campaniform sensilla activity can affect the magnitude and timing of muscle contractions. [5]