Related Research Articles

Carbon dioxide is a chemical compound with the chemical formula CO2. It is made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature, and as the source of available carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. When carbon dioxide dissolves in water, it forms carbonate and mainly bicarbonate, which causes ocean acidification as atmospheric CO2 levels increase.

Global Warming Potential (GWP) is an index to measure how much infrared thermal radiation a greenhouse gas would absorb over a given time frame after it has been added to the atmosphere. The GWP makes different greenhouse gases comparable with regard to their "effectiveness in causing radiative forcing". It is expressed as a multiple of the radiation that would be absorbed by the same mass of added carbon dioxide, which is taken as a reference gas. Therefore, the GWP has a value of 1 for CO2. For other gases it depends on how strongly the gas absorbs infrared thermal radiation, how quickly the gas leaves the atmosphere, and the time frame being considered.

Photosynthesis is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their activities. Photosynthetic organisms use intracellular organic compounds to store the chemical energy they produce in photosynthesis within organic compounds like sugars, glycogen, cellulose and starches. Photosynthesis is usually used to refer to oxygenic photosynthesis, a process that produces oxygen. To use this stored chemical energy, the organisms' cells metabolize the organic compounds through another process called cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth.
Svante August Arrhenius was a Swedish scientist. Originally a physicist, but often referred to as a chemist, Arrhenius was one of the founders of the science of physical chemistry. He received the Nobel Prize for Chemistry in 1903, becoming the first Swedish Nobel laureate. In 1905, he became the director of the Nobel Institute, where he remained until his death.

Dry ice colloquially means the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimes directly from the solid state to the gas state. It is used primarily as a cooling agent, but is also used in fog machines at theatres for dramatic effects. Its advantages include lower temperature than that of water ice and not leaving any residue (other than incidental frost from moisture in the atmosphere). It is useful for preserving frozen foods (such as ice cream) where mechanical cooling is unavailable.

Steelmaking is the process of producing steel from iron ore and/or scrap. In steelmaking, impurities such as nitrogen, silicon, phosphorus, sulfur and excess carbon are removed from the sourced iron, and alloying elements such as manganese, nickel, chromium, carbon and vanadium are added to produce different grades of steel.

Ethanolamine is a naturally occurring organic chemical compound with the formula HOCH
2CH
2NH
2 or C
2H
7NO. The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from the expansion of a hot gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have their efficiency limited by the Carnot efficiency and therefore produce waste heat.

Modified atmosphere packaging (MAP) is the practice of modifying the composition of the internal atmosphere of a package in order to improve the shelf life. The need for this technology for food arises from the short shelf life of food products such as meat, fish, poultry, and dairy in the presence of oxygen. In food, oxygen is readily available for lipid oxidation reactions. Oxygen also helps maintain high respiration rates of fresh produce, which contribute to shortened shelf life. From a microbiological aspect, oxygen encourages the growth of aerobic spoilage microorganisms. Therefore, the reduction of oxygen and its replacement with other gases can reduce or delay oxidation reactions and microbiological spoilage. Oxygen scavengers may also be used to reduce browning due to lipid oxidation by halting the auto-oxidative chemical process. Besides, MAP changes the gaseous atmosphere by incorporating different compositions of gases.

Cultivation of cannabis is the production of cannabis infructescences. Cultivation techniques for other purposes differ.

Carbon capture and storage (CCS) is a process in which a relatively pure stream of carbon dioxide (CO2) from industrial sources is separated, treated and transported to a long-term storage location. For example, the burning of fossil fuels or biomass results in a stream of CO2 that could be captured and stored by CCS. Usually the CO2 is captured from large point sources, such as a chemical plant or a bioenergy plant, and then stored in a suitable geological formation. The aim is to reduce greenhouse gas emissions and thus mitigate climate change. For example, CCS retrofits for existing power plants can be one of the ways to limit emissions from the electricity sector and meet the Paris Agreement goals.
A carbon dioxide sensor or CO2 sensor is an instrument for the measurement of carbon dioxide gas. The most common principles for CO2 sensors are infrared gas sensors (NDIR) and chemical gas sensors. Measuring carbon dioxide is important in monitoring indoor air quality, the function of the lungs in the form of a capnograph device, and many industrial processes.
Carbon dioxide hydrate or carbon dioxide clathrate is a snow-like crystalline substance composed of water ice and carbon dioxide. It normally is a Type I gas clathrate. There has also been some experimental evidence for the development of a metastable Type II phase at a temperature near the ice melting point. The clathrate can exist below 283K (10 °C) at a range of pressures of carbon dioxide. CO2 hydrates are widely studied around the world due to their promising prospects of carbon dioxide capture from flue gas and fuel gas streams relevant to post-combustion and pre-combustion capture. It is also quite likely to be important on Mars due to the presence of carbon dioxide and ice at low temperatures.

Greenhouse gas (GHG) emissions from human activities intensify the greenhouse effect. This contributes to climate change. Carbon dioxide, from burning fossil fuels such as coal, oil, and natural gas, is one of the most important factors in causing climate change. The largest emitters are China followed by the United States. The United States has higher emissions per capita. The main producers fueling the emissions globally are large oil and gas companies. Emissions from human activities have increased atmospheric carbon dioxide by about 50% over pre-industrial levels. The growing levels of emissions have varied, but have been consistent among all greenhouse gases. Emissions in the 2010s averaged 56 billion tons a year, higher than any decade before. Total cumulative emissions from 1870 to 2017 were 425±20 GtC from fossil fuels and industry, and 180±60 GtC from land use change. Land-use change, such as deforestation, caused about 31% of cumulative emissions over 1870–2017, coal 32%, oil 25%, and gas 10%.
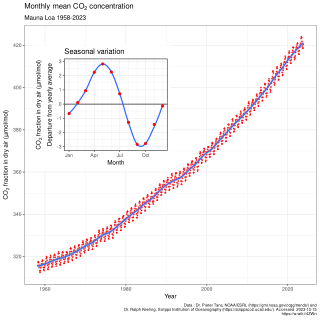
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of several greenhouse gases in the atmosphere of Earth. The current global average concentration of carbon dioxide (CO2) in the atmosphere is 421 ppm as of May 2022 (0.04%). This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century. The increase is due to human activity.

A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change.
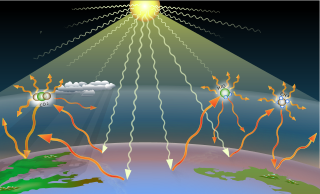
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F).
Lower-temperature fuel cell types such as the proton exchange membrane fuel cell, phosphoric acid fuel cell, and alkaline fuel cell require pure hydrogen as fuel, typically produced from external reforming of natural gas. However, fuels cells operating at high temperature such as the solid oxide fuel cell (SOFC) are not poisoned by carbon monoxide and carbon dioxide, and in fact can accept hydrogen, carbon monoxide, carbon dioxide, steam, and methane mixtures as fuel directly, because of their internal shift and reforming capabilities. This opens up the possibility of efficient fuel cell-based power cycles consuming solid fuels such as coal and biomass, the gasification of which results in syngas containing mostly hydrogen, carbon monoxide and methane which can be cleaned and fed directly to the SOFCs without the added cost and complexity of methane reforming, water gas shifting and hydrogen separation operations which would otherwise be needed to isolate pure hydrogen as fuel. A power cycle based on gasification of solid fuel and SOFCs is called an Integrated Gasification Fuel Cell (IGFC) cycle; the IGFC power plant is analogous to an integrated gasification combined cycle power plant, but with the gas turbine power generation unit replaced with a fuel cell power generation unit. By taking advantage of intrinsically high energy efficiency of SOFCs and process integration, exceptionally high power plant efficiencies are possible. Furthermore, SOFCs in the IGFC cycle can be operated so as to isolate a carbon dioxide-rich anodic exhaust stream, allowing efficient carbon capture to address greenhouse gas emissions concerns of coal-based power generation.
References
- 1 2 George F. Van Patten; Alyssa F. Bust; Tom LaSpina (January 1997). Gardening Indoors with CO2. Van Patten Publishing. pp. 28–. ISBN 978-1-878823-19-9.
- ↑ Keith Roberto (1 August 2003). How-to Hydroponics. Futuregarden, Inc. pp. 39–. ISBN 978-0-9672026-1-7.
- ↑ Karen Lovett March 26, 2009 Publication: Telegraph, The (Nashua, NH)
- ↑ Jorge Cervantes (2002). Indoor Marijuana Horticulture. Van Patten Publishing. pp. 195–. ISBN 978-1-878823-29-8.
- ↑ Rosenthal, E. (2003). The Best of Ask Ed: Your Marijuana Questions Answered. Quick American. p. 83. ISBN 9780932551573 . Retrieved 2015-09-14.