
Hydrogen peroxide is a chemical compound with the formula H2O2. In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution in water for consumer use and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used as both a monopropellant and an oxidizer in rocketry.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colourless and odourless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

A turbopump is a propellant pump with two main components: a rotodynamic pump and a driving gas turbine, usually both mounted on the same shaft, or sometimes geared together. They were initially developed in Germany in the early 1940s. The purpose of a turbopump is to produce a high-pressure fluid for feeding a combustion chamber or other use. While other use cases exist, they are most commonly found in liquid rocket engines.

A hybrid-propellant rocket is a rocket with a rocket motor that uses rocket propellants in two different phases: one solid and the other either gas or liquid. The hybrid rocket concept can be traced back to the early 1930s.
A monopropellant rocket is a rocket that uses a single chemical as its propellant. Monopropellant rockets are commonly used as small attitude and trajectory control rockets in satellites, rocket upper stages, manned spacecraft, and spaceplanes.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate.

A hypergolic propellant is a rocket propellant combination used in a rocket engine, whose components spontaneously ignite when they come into contact with each other.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.
T-Stoff (; 'substance T') was a stabilised high test peroxide used in Germany during World War II. T-Stoff was specified to contain 80% (occasionally 85%) hydrogen peroxide (H2O2), remainder water, with traces (<0.1%) of stabilisers. Stabilisers used included 0.0025% phosphoric acid, a mixture of phosphoric acid, sodium phosphate and 8-oxyquinoline, and sodium stannate.

A liquid-propellant rocket or liquid rocket uses a rocket engine burning liquid propellants. (Alternate approaches use gaseous or solid propellants.) Liquids are desirable propellants because they have reasonably high density and their combustion products have high specific impulse (Isp). This allows the volume of the propellant tanks to be relatively low.

The J-2, commonly known as Rocketdyne J-2, was a liquid-fuel cryogenic rocket engine used on NASA's Saturn IB and Saturn V launch vehicles. Built in the United States by Rocketdyne, the J-2 burned cryogenic liquid hydrogen (LH2) and liquid oxygen (LOX) propellants, with each engine producing 1,033.1 kN (232,250 lbf) of thrust in vacuum. The engine's preliminary design dates back to recommendations of the 1959 Silverstein Committee. Rocketdyne won approval to develop the J-2 in June 1960 and the first flight, AS-201, occurred on 26 February 1966. The J-2 underwent several minor upgrades over its operational history to improve the engine's performance, with two major upgrade programs, the de Laval nozzle-type J-2S and aerospike-type J-2T, which were cancelled after the conclusion of the Apollo program.
High-test peroxide (HTP) is a highly concentrated solution of hydrogen peroxide, with the remainder consisting predominantly of water. In contact with a catalyst, it decomposes into a high-temperature mixture of steam and oxygen, with no remaining liquid water. It was used as a propellant of HTP rockets and torpedoes, and has been used for high-performance vernier engines.
The highest specific impulse chemical rockets use liquid propellants. They can consist of a single chemical or a mix of two chemicals, called bipropellants. Bipropellants can further be divided into two categories; hypergolic propellants, which ignite when the fuel and oxidizer make contact, and non-hypergolic propellants which require an ignition source.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is acutely poisonous.

The staged combustion cycle is a power cycle of a bipropellant rocket engine. In the staged combustion cycle, propellant flows through multiple combustion chambers, and is thus combusted in stages. The main advantage relative to other rocket engine power cycles is high fuel efficiency, measured through specific impulse, while its main disadvantage is engineering complexity.

Chemical decomposition, or chemical breakdown, is the process or effect of simplifying a single chemical entity into two or more fragments. Chemical decomposition is usually regarded and defined as the exact opposite of chemical synthesis. In short, the chemical reaction in which two or more products are formed from a single reactant is called a decomposition reaction.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
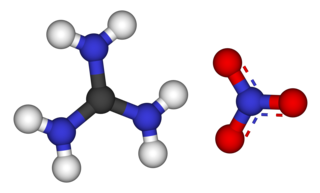
Guanidine nitrate is the chemical compound with the formula [C(NH2)3]NO3. It is a colorless, water-soluble salt. It is produced on a large scale and finds use as precursor for nitroguanidine, fuel in pyrotechnics and gas generators. Its correct name is guanidinium nitrate, but the colloquial term guanidine nitrate is widely used.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Rocket propellant is used as reaction mass ejected from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical rocket, or from an external source, as with ion engines.


















