Bioleaching is the extraction or liberation of metals from their ores through the use of living organisms. Bioleaching is one of several applications within biohydrometallurgy and several methods are used to treat ores or concentrates containing copper, zinc, lead, arsenic, antimony, nickel, molybdenum, gold, silver, and cobalt.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, washing, concentration, separation, chemical processes and extraction of pure metal and their alloying to suit various applications, sometimes for direct use as a finished product, but more often in a form that requires further working to achieve the given properties to suit the applications.

Chalcopyrite ( KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.
Gold cyanidation is a hydrometallurgical technique for extracting gold from low-grade ore by converting the gold to a water-soluble coordination complex. It is the most commonly used leaching process for gold extraction. Cyanidation is also widely used in the extraction of silver, usually after froth flotation.

Copper extraction refers to the methods used to obtain copper from its ores. The conversion of copper ores consists of a series of physical, chemical and electrochemical processes. Methods have evolved and vary with country depending on the ore source, local environmental regulations, and other factors.

Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic. This is used in mineral processing, paper recycling and waste-water treatment industries. Historically this was first used in the mining industry, where it was one of the great enabling technologies of the 20th century. It has been described as "the single most important operation used for the recovery and upgrading of sulfide ores". The development of froth flotation has improved the recovery of valuable minerals, such as copper- and lead-bearing minerals. Along with mechanized mining, it has allowed the economic recovery of valuable metals from much lower-grade ore than previously.

Mineral processing is the process of separating commercially valuable minerals from their ores in the field of extractive metallurgy. Depending on the processes used in each instance, it is often referred to as ore dressing or ore milling.
Hydrometallurgy is a technique within the field of extractive metallurgy, the obtaining of metals from their ores. Hydrometallurgy involve the use of aqueous solutions for the recovery of metals from ores, concentrates, and recycled or residual materials. Processing techniques that complement hydrometallurgy are pyrometallurgy, vapour metallurgy, and molten salt electrometallurgy. Hydrometallurgy is typically divided into three general areas:

Gold extraction is the extraction of gold from dilute ores using a combination of chemical processes. Gold mining produces about 3600 tons annually, and another 300 tons is produced from recycling.
The Merrill–Crowe Process is a separation technique for removing gold from the solution obtained by the cyanide leaching of gold ores. It is an improvement of the MacArthur-Forrest process, where an additional vacuum is managed to remove air in the solution, and zinc dust is used instead of zinc shavings.

Electrowinning, also called electroextraction, is the electrodeposition of metals from their ores that have been put in solution via a process commonly referred to as leaching. Electrorefining uses a similar process to remove impurities from a metal. Both processes use electroplating on a large scale and are important techniques for the economical and straightforward purification of non-ferrous metals. The resulting metals are said to be electrowon.

Heap leaching is an industrial mining process used to extract precious metals, copper, uranium, and other compounds from ore using a series of chemical reactions that absorb specific minerals and re-separate them after their division from other earth materials. Similar to in situ mining, heap leach mining differs in that it places ore on a liner, then adds the chemicals via drip systems to the ore, whereas in situ mining lacks these liners and pulls pregnant solution up to obtain the minerals. Heap leaching is widely used in modern large-scale mining operations as it produces the desired concentrates at a lower cost compared to conventional processing methods such as flotation, agitation, and vat leaching.
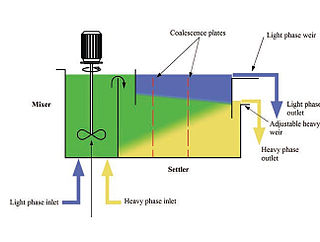
Mixer settlers are a class of mineral process equipment used in the solvent extraction process. A mixer settler consists of a first stage that mixes the phases together followed by a quiescent settling stage that allows the phases to separate by gravity.

In-situ leaching (ISL), also called in-situ recovery (ISR) or solution mining, is a mining process used to recover minerals such as copper and uranium through boreholes drilled into a deposit, in situ. In-situ leach works by artificially dissolving minerals occurring naturally in the solid state.
In metallurgical processes tank leaching is a hydrometallurgical method of extracting valuable material from ore.
Leaching is a process widely used in extractive metallurgy where ore is treated with chemicals to convert the valuable metals within the ore, into soluble salts while the impurity remains insoluble. These can then be washed out and processed to give the pure metal; the materials left over are commonly known as tailings. Compared to pyrometallurgy, leaching is easier to perform, requires less energy and is potentially less harmful as no gaseous pollution occurs. Drawbacks of leaching include its lower efficiency and the often significant quantities of waste effluent and tailings produced, which are usually either highly acidic or alkali as well as toxic.

Cobalt extraction refers to the techniques used to extract cobalt from its ores and other compound ores. Several methods exist for the separation of cobalt from copper and nickel. They depend on the concentration of cobalt and the exact composition of the ore used.
The International Cyanide Management Code for the Manufacture, Transport and Use of Cyanide in the Production of Gold, commonly referred to as the Cyanide Code, is a voluntary program designed to assist the global gold and silver mining industries and the producers and transporters of cyanide used in gold and silver mining in improving cyanide management practices and to publicly demonstrate their compliance with the Cyanide Code through an independent and transparent process. The Cyanide Code is intended to reduce the potential exposure of workers and communities to harmful concentrations of cyanide‚ limit releases of cyanide to the environment‚ and enhance response actions in the event of an exposure or release.

The Jameson Cell is a high-intensity froth flotation cell that was invented by Laureate Professor Graeme Jameson of the University of Newcastle (Australia) and developed in conjunction with Mount Isa Mines Limited.
Mineral processing and extraction of metals are very energy-intensive processes, which are not exempted of producing large volumes of solid residues and wastewater, which also require energy to be further treated and disposed. Moreover, as the demand for metals increases, the metallurgical industry must rely on sources of materials with lower metal contents both from a primary and/or secondary raw materials. Consequently, mining activities and waste recycling must evolve towards the development of more selective, efficient and environmentally friendly mineral and metal processing routes.










