Related Research Articles

Ischemia or ischaemia is a restriction in blood supply to any tissue, muscle group, or organ of the body, causing a shortage of oxygen that is needed for cellular metabolism. Ischemia is generally caused by problems with blood vessels, with resultant damage to or dysfunction of tissue i.e. hypoxia and microvascular dysfunction. It also implies local hypoxia in a part of a body resulting from constriction. Ischemia causes not only insufficiency of oxygen, but also reduced availability of nutrients and inadequate removal of metabolic wastes. Ischemia can be partial or total blockage. The inadequate delivery of oxygenated blood to the organs must be resolved either by treating the cause of the inadequate delivery or reducing the oxygen demand of the system that needs it. For example, patients with myocardial ischemia have a decreased blood flow to the heart and are prescribed with medications that reduce chronotrophy and ionotrophy to meet the new level of blood delivery supplied by the stenosed vasculature so that it is adequate.

Reperfusion injury, sometimes called ischemia-reperfusion injury (IRI) or reoxygenation injury, is the tissue damage caused when blood supply returns to tissue after a period of ischemia or lack of oxygen. The absence of oxygen and nutrients from blood during the ischemic period creates a condition in which the restoration of circulation results in inflammation and oxidative damage through the induction of oxidative stress rather than restoration of normal function.

Lipid emulsion or fat emulsion refers to an emulsion of fat for human intravenous use, to administer nutrients to critically-ill patients that cannot consume food. It is often referred to by the brand name of the most commonly used version, Intralipid, which is an emulsion containing soybean oil, egg phospholipids and glycerin, and is available in 10%, 20% and 30% concentrations. The 30% concentration is not approved for direct intravenous infusion, but should be mixed with amino acids and dextrose as part of a total nutrient admixture.

Nicorandil is a vasodilatory drug used to treat angina.
Myocardial stunning or transient post-ischemic myocardial dysfunction is a state of mechanical cardiac dysfunction that can occur in a portion of myocardium without necrosis after a brief interruption in perfusion, despite the timely restoration of normal coronary blood flow. In this situation, even after ischemia has been relieved and myocardial blood flow (MBF) returns to normal, myocardial function is still depressed for a variable period of time, usually days to weeks. This reversible reduction of function of heart contraction after reperfusion is not accounted for by tissue damage or reduced blood flow, but rather, its thought to represent a perfusion-contraction "mismatch". Myocardial stunning was first described in laboratory canine experiments in the 1970s where LV wall abnormalities were observed following coronary artery occlusion and subsequent reperfusion.
Ischemic preconditioning (IPC) is an experimental technique for producing resistance to the loss of blood supply, and thus oxygen, to tissues of many types. In the heart, IPC is an intrinsic process whereby repeated short episodes of ischaemia protect the myocardium against a subsequent ischaemic insult. It was first identified in 1986 by Murry et al. This group exposed anesthetised open-chest dogs to four periods of 5 minute coronary artery occlusions followed by a 5-minute period of reperfusion before the onset of a 40-minute sustained occlusion of the coronary artery. The control animals had no such period of “ischaemic preconditioning” and had much larger infarct sizes compared with the dogs that did. The exact molecular pathways behind this phenomenon have yet to be fully understood.
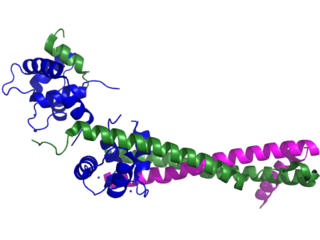
Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to actin in relaxed muscle. When calcium binds to the troponin C, it causes conformational changes which lead to dislocation of troponin I. Afterwards, tropomyosin leaves the binding site for myosin on actin leading to contraction of muscle. The letter I is given due to its inhibitory character. It is a useful marker in the laboratory diagnosis of heart attack. It occurs in different plasma concentration but the same circumstances as troponin T - either test can be performed for confirmation of cardiac muscle damage and laboratories usually offer one test or the other.

Myocardial perfusion imaging or scanning is a nuclear medicine procedure that illustrates the function of the heart muscle (myocardium).
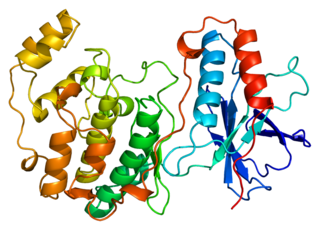
Mitogen-activated protein kinase 14, also called p38-α, is an enzyme that in humans is encoded by the MAPK14 gene.

Protein kinase C epsilon type (PKCε) is an enzyme that in humans is encoded by the PRKCE gene. PKCε is an isoform of the large PKC family of protein kinases that play many roles in different tissues. In cardiac muscle cells, PKCε regulates muscle contraction through its actions at sarcomeric proteins, and PKCε modulates cardiac cell metabolism through its actions at mitochondria. PKCε is clinically significant in that it is a central player in cardioprotection against ischemic injury and in the development of cardiac hypertrophy.

Acadesine (INN), also known as 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, AICA-riboside, and AICAR, is an AMP-activated protein kinase activator which is used for the treatment of acute lymphoblastic leukemia and may have applications in treating other disorders such as diabetes. AICAR has been used clinically to treat and protect against cardiac ischemic injury. The drug was first used in the 1980s as a method to preserve blood flow to the heart during surgery.

Contraction band necrosis is a type of uncontrolled cell death (necrosis) unique to cardiac myocytes and thought to arise in reperfusion from hypercontraction, which results in sarcolemmal rupture.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon and/or stents to open up the artery. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.
A diagnosis of myocardial infarction is created by integrating the history of the presenting illness and physical examination with electrocardiogram findings and cardiac markers. A coronary angiogram allows visualization of narrowings or obstructions on the heart vessels, and therapeutic measures can follow immediately. At autopsy, a pathologist can diagnose a myocardial infarction based on anatomopathological findings.

Cariporide is a selective Na+/H+ exchange inhibitor. Cariporide has been shown to actively suppress the cell death caused by oxidative stress.
Survivor Activating Factor Enhancement (SAFE) is a metabolic pathway. It is an intrinsic protective signaling programme to limit cell death activated by the heart. This pathway allows ischaemic postconditioning that helps protect against reperfusion injury. This path involves the activation of a transcription factor called signal transducer and activator of transcription 3 (STAT3). The SAFE pathway interacts with the reperfusion injury salvage kinase pathway to convey the ischemic postconditioning stimulus from the cell surface to the mitochondria, where many of the prosurvival and death signals appear to converge.

Rottlerin (mallotoxin) is a polyphenol natural product isolated from the Asian tree Mallotus philippensis. Rottlerin displays a complex spectrum of pharmacology.
Remote ischemic conditioning (RIC) is an experimental medical procedure that aims to reduce the severity of ischaemic injury to an organ such as the heart or the brain, most commonly in the situation of a heart attack or a stroke, or during procedures such as heart surgery when the heart may temporary suffer ischaemia during the operation, by triggering the body's natural protection against tissue injury. Although noted to have some benefits in experimental models in animals, this is still an experimental procedure in humans and initial evidence from small studies have not been replicated in larger clinical trials. Successive clinical trials have failed to identify evidence supporting a protective role in humans.
Gerd Heusch is a German physician, physiologist, and professor as well as chair of the Institute for Pathophysiology at the University of Essen Medical School.
Roberta Anne Gottlieb is an American oncologist, academic, and researcher. She is a Professor, and Vice-Chair of Translational Medicine in the Department of Biomedical Sciences at Cedars-Sinai Medical Center, and a Professor of Medicine at the University of California, Los Angeles.
References
- ↑ Kübler, W. (April 1996). "Cardioprotection: definition, classification, and fundamental principles". Heart. 75 (4): 330–333. doi:10.1136/hrt.75.4.330. PMC 484304 . PMID 8705755.
- 1 2 Vinten-Johansen, J; Shi, W (2011). "Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions". Journal of Cardiovascular Pharmacology and Therapeutics. 16 (3–4): 260–6. doi:10.1177/1074248411415270. PMID 21821526. S2CID 20432309.
- ↑ Murry, CE; Jennings, RB; Reimer, KA (November 1986). "Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium". Circulation. 74 (5): 1124–36. doi: 10.1161/01.cir.74.5.1124 . PMID 3769170.
- ↑ Hausenloy, DJ; Yellon, DM (15 February 2004). "New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway". Cardiovascular Research. 61 (3): 448–60. doi: 10.1016/j.cardiores.2003.09.024 . PMID 14962476.
- ↑ Kuzuya, T; Hoshida, S; Yamashita, N; Fuji, H; Oe, H; Hori, M; Kamada, T; Tada, M (June 1993). "Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia". Circulation Research. 72 (6): 1293–9. doi: 10.1161/01.res.72.6.1293 . PMID 8495557.
- ↑ Marber, MS; Latchman, DS; Walker, JM; Yellon, DM (September 1993). "Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction". Circulation. 88 (3): 1264–72. doi: 10.1161/01.cir.88.3.1264 . PMID 8353888.
- ↑ Bolli, R (24 November 2000). "The late phase of preconditioning". Circulation Research. 87 (11): 972–83. doi: 10.1161/01.res.87.11.972 . PMID 11090541.
- ↑ Bolli, R; Li, QH; Tang, XL; Guo, Y; Xuan, YT; Rokosh, G; Dawn, B (December 2007). "The late phase of preconditioning and its natural clinical application--gene therapy". Heart Failure Reviews. 12 (3–4): 189–99. doi:10.1007/s10741-007-9031-4. PMC 3652384 . PMID 17541820.
- ↑ Przyklenk, K; Bauer, B; Ovize, M; Kloner, RA; Whittaker, P (March 1993). "Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion". Circulation. 87 (3): 893–9. doi: 10.1161/01.cir.87.3.893 . PMID 7680290.
- ↑ Zhao, ZQ; Corvera, JS; Halkos, ME; Kerendi, F; Wang, NP; Guyton, RA; Vinten-Johansen, J (August 2003). "Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning". American Journal of Physiology. Heart and Circulatory Physiology. 285 (2): H579-88. doi:10.1152/ajpheart.01064.2002. PMID 12860564.
- ↑ Kerendi, F; Kin, H; Halkos, ME; Jiang, R; Zatta, AJ; Zhao, ZQ; Guyton, RA; Vinten-Johansen, J (September 2005). "Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors". Basic Research in Cardiology. 100 (5): 404–12. doi:10.1007/s00395-005-0539-2. PMID 15965583. S2CID 34810140.
- ↑ Wolfrum, S; Schneider, K; Heidbreder, M; Nienstedt, J; Dominiak, P; Dendorfer, A (15 August 2002). "Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform". Cardiovascular Research. 55 (3): 583–9. doi:10.1016/s0008-6363(02)00408-x. PMID 12160956.
- ↑ Weinbrenner, C; Nelles, M; Herzog, N; Sárváry, L; Strasser, RH (15 August 2002). "Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway". Cardiovascular Research. 55 (3): 590–601. doi: 10.1016/s0008-6363(02)00446-7 . PMID 12160957.
- ↑ Zatta, AJ; Kin, H; Lee, G; Wang, N; Jiang, R; Lust, R; Reeves, JG; Mykytenko, J; Guyton, RA; Zhao, ZQ; Vinten-Johansen, J (1 May 2006). "Infarct-sparing effect of myocardial postconditioning is dependent on protein kinase C signalling". Cardiovascular Research. 70 (2): 315–24. doi: 10.1016/j.cardiores.2005.11.030 . PMID 16443207.
- ↑ Philipp, S; Yang, XM; Cui, L; Davis, AM; Downey, JM; Cohen, MV (1 May 2006). "Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade". Cardiovascular Research. 70 (2): 308–14. doi:10.1016/j.cardiores.2006.02.014. PMID 16545350.
- ↑ Zhong, GQ; Tu, RH; Zeng, ZY; Li, QJ; He, Y; Li, S; He, Y; Xiao, F (15 June 2014). "Novel functional role of heat shock protein 90 in protein kinase C-mediated ischemic postconditioning". The Journal of Surgical Research. 189 (2): 198–206. doi:10.1016/j.jss.2014.01.038. PMID 24742623.