
An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have been synthesized from most transition metals and f-block metals, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as =CR2, carbene ligands are intermediate between alkyls (−CR3) and carbynes (≡CR). Many different carbene-based reagents such as Tebbe's reagent are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Vanadium(III) chloride describes the inorganic compound with the formula VCl3 and its hydrates. It forms a purple anhydrous form and a green hexahydrate [VCl2(H2O)4]Cl·2H2O. These hygroscopic salts are common precursors to other vanadium(III) complexes and is used as a mild reducing agent.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.

Organosilver chemistry is the study of organometallic compounds containing a carbon to silver chemical bond. The theme is less developed than organocopper chemistry.
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
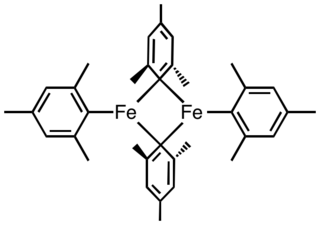
Tetramesityldiiron is an organoiron compound with the formula Fe2(C6H2(CH3)3)4. It is a red, air-sensitive solid that is used as a precursor to other iron complexes. It adopts a centrosymmetric structure. The complex is a Lewis acid, forming monomeric adducts, e.g. Fe(C6H2(CH3)3)2pyridine2. The complex is prepared by treating ferrous halides with the Grignard reagent formed from mesityl bromide:

Marinella Mazzanti is an Italian inorganic chemist specialized in coordination chemistry. She is a professor at EPFL and the head of the group of Coordination Chemistry at EPFL's School of Basic Sciences.

Transition metal carbonate and bicarbonate complexes are coordination compounds containing carbonate (CO32-) and bicarbonate (HCO3-) as ligands. The inventory of complexes is large, enhanced by the fact that the carbonate ligand can bind metal ions in a variety of bonding modes. They illustrate the fate of low valent complexes when exposed to air.

trans-Dicarbonyldi-μ-chlorodichlorodiplatinum is the first ever isolated metal-carbonyl complex. It was first synthesized by Paul Schützenberger in 1868. This discovery of [Pt(CO)Cl2]2 opened the door for the exploration of metal-carbonyl complexes and represents a significant breakthrough in the field of organometallic chemistry. Though [Pt(CO)Cl2]2 itself is not used in industry, metal carbonyls are an important class of catalysts for a wide range of industrial processes including hydroformylation amongst others. As such, the discovery of [Pt(CO)Cl2]2 was an important milestone both in the synthesis of organometallic compounds and in the development of many important catalysts widely used today.
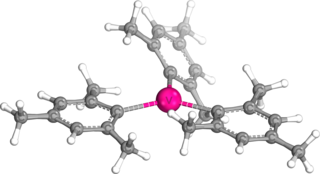
Trimesitylvanadium (mesityl or Mes = 2,4,6-trimethylphenyl) is one of the organovanadium complexes with vanadium in an oxidation state of 3. This compound was first synthesized by W. Seidel and G. Kreisel in 1974. To prepare this compound, VCl3(THF)3 (THF = tetrahydrofuran) was reacted with Grignard reagent MesMgBr to form a blue solution at room temperature. It is precipitated by the addition of dioxane, which results in a blue solid. It is thermally stable, but it is also an air-sensitive compound.
A metal-formaldehyde complex is a coordination complex in which a formaldehyde ligand has two bonds to the metal atom(s) (η2-CH2O). This type of ligand has been reported in both monometallic and bimetallic complexes.

Fausto Calderazzo was an Italian inorganic chemist. He gained renown from numerous contributions in inorganic chemistry and organometallic chemistry. Hr was born in Parma, on March 8, 1930, where his father served in the Royal Italian army. He died in Pisa on June 1, 2014, at the age of 84.

Luigi Sacconi was an Italian inorganic chemist who gained renown for contributions to coordination chemistry. He was born on February 28, 1911 in S. Croce sull'Arno. He died September 1, 1992. He received a Doctor of Pharmacy at the University of Florence. He was on the faculties at the University of Parma, Turin, and then Florence. He was mentor of future influential inorganic chemists including Ivano Bertini, Claudio Bianchini, Fausto Calderazzo, Carlo Floriani, Dante Gatteschi, Carlo Mealli, and Maurizio Peruzzini. Among his many contributions, Sacconi popularized tripodal ligands, which often stabilize pentacoordinate complexes with unusual electronic or chemical properties.















