In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge.
A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have been synthesized from most transition metals and f-block metals, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as =CR2, carbene ligands are intermediate between alkyls (−CR3) and carbynes (≡CR). Many different carbene-based reagents such as Tebbe's reagent are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.
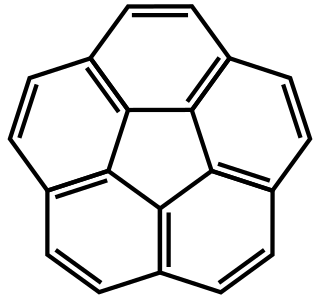
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.

Gold(I,III) chloride is the inorganic compound with the chemical formula Au4Cl8. It is an example of a mixed valence compound as it contains gold in two oxidation states; square-planar gold(III) and almost linear gold(I). The compound, which is black, is photosensitive as well as air- and moisture-sensitive.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

Group 2 organometallic chemistry refers to the organic derivativess of any group 2 element. It is a subtheme to main group organometallic chemistry. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are typically limited to academic interests.

Organocerium chemistry is the science of organometallic compounds that contain one or more chemical bond between carbon and cerium. These compounds comprise a subset of the organolanthanides. Most organocerium compounds feature Ce(III) but some Ce(IV) derivatives are known.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
Metal arene complexes are organometallic compounds of the formula (C6R6)xMLy. Common classes are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis. The principles that describe arene complexes extend to related organic ligands such as many heterocycles (e.g. thiophene) and polycyclic aromatic compounds (e.g. naphthalene).

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.
A Fischer carbene is a type of transition metal carbene complex, which is an organometallic compound containing a divalent organic ligand. In a Fischer carbene, the carbene ligand is a σ-donor π-acceptor ligand. Because π-backdonation from the metal centre is generally weak, the carbene carbon is electrophilic.
Germyl, trihydridogermanate(1-), trihydrogermanide, trihydridogermyl or according to IUPAC Red Book: germanide is an anion containing germanium bounded with three hydrogens, with formula GeH−3. Germyl is the IUPAC term for the –GeH3 group. For less electropositive elements the bond can be considered covalent rather than ionic as "germanide" indicates. Germanide is the base for germane when it loses a proton.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.
Arene complexes of univalent gallium, indium, and thallium are complexes featuring the centric (η6) coordination of the metal to the arene. Although arene complexes of transitional metals have long been reported, arene complexes of the main group elements remain scarce. This might be partly explained by the difference in energy of the d and p orbitals.

trans-Dicarbonyldi-μ-chlorodichlorodiplatinum is the first ever isolated metal-carbonyl complex. It was first synthesized by Paul Schützenberger in 1868. This discovery of [Pt(CO)Cl2]2 opened the door for the exploration of metal-carbonyl complexes and represents a significant breakthrough in the field of organometallic chemistry. Though [Pt(CO)Cl2]2 itself is not used in industry, metal carbonyls are an important class of catalysts for a wide range of industrial processes including hydroformylation amongst others. As such, the discovery of [Pt(CO)Cl2]2 was an important milestone both in the synthesis of organometallic compounds and in the development of many important catalysts widely used today.
A hydrotelluride or tellanide is an ion or a chemical compound containing the [HTe]− anion which has a hydrogen atom connected to a tellurium atom. HTe is a pseudohalogen. Organic compounds containing the -TeH group are called tellurols. "Tellanide" is the IUPAC name from the Red Book, but hydrogen(tellanide)(1−) is also listed. "Tellanido" as a ligand is not named, however ditellanido is used for HTeTe−.
Carlo Floriani was an inorganic chemist who gained renown for contributions to organometallic and coordination chemistry. He was born in Casalmaggiore, Cremona (Italy) in 1940 and died in 2005. He held positions at the University of Pisa, Columbia University, and Ecole Polytechnique, Lausanne. He had been a protoge of Fausto Calderazzo.

![Figure 4. Crystallographic structure of [H(THF)2][Nb2(m-Cl)3(CO)8] Crystallographic structure of (H(THF)2)(Nb2(m-Cl)3(CO)8).png](http://upload.wikimedia.org/wikipedia/commons/thumb/1/11/Crystallographic_structure_of_%28H%28THF%292%29%28Nb2%28%CE%BC-Cl%293%28CO%298%29.png/225px-Crystallographic_structure_of_%28H%28THF%292%29%28Nb2%28%CE%BC-Cl%293%28CO%298%29.png)













