
Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.

The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years, but the combustion of cellulose in a fire is a reaction that takes place in fractions of a second. For most reactions, the rate decreases as the reaction proceeds. A reaction's rate can be determined by measuring the changes in concentration over time.
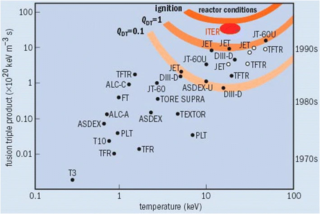
The Lawson criterion is a figure of merit used in nuclear fusion research. It compares the rate of energy being generated by fusion reactions within the fusion fuel to the rate of energy losses to the environment. When the rate of production is higher than the rate of loss, the system will produce net energy. If enough of that energy is captured by the fuel, the system will become self-sustaining and is said to be ignited.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
The Rosenmund reduction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918.

Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
Photon polarization is the quantum mechanical description of the classical polarized sinusoidal plane electromagnetic wave. An individual photon can be described as having right or left circular polarization, or a superposition of the two. Equivalently, a photon can be described as having horizontal or vertical linear polarization, or a superposition of the two.
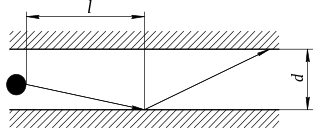
In physics, Knudsen diffusion, named after Martin Knudsen, is a means of diffusion that occurs when the scale length of a system is comparable to or smaller than the mean free path of the particles involved. An example of this is in a long pore with a narrow diameter (2–50 nm) because molecules frequently collide with the pore wall. As another example, consider the diffusion of gas molecules through very small capillary pores. If the pore diameter is smaller than the mean free path of the diffusing gas molecules, and the density of the gas is low, the gas molecules collide with the pore walls more frequently than with each other, leading to Knudsen diffusion.
In chemistry, phase-boundary catalysis (PBC) is a type of heterogeneous catalytic system which facilitates the chemical reaction of a particular chemical component in an immiscible phase to react on a catalytic active site located at a phase boundary. The chemical component is soluble in one phase but insoluble in the other. The catalyst for PBC has been designed in which the external part of the zeolite is hydrophobic, internally it is usually hydrophilic, notwithstanding to polar nature of some reactants. In this sense, the medium environment in this system is close to that of an enzyme. The major difference between this system and enzyme is lattice flexibility. The lattice of zeolite is rigid, whereas the enzyme is flexible.
The Weisz–Prater criterion is a method used to estimate the influence of pore diffusion on reaction rates in heterogeneous catalytic reactions. If the criterion is satisfied, pore diffusion limitations are negligible. The criterion is
Where is the reaction rate per volume of catalyst, is the catalyst particle radius, is the reactant concentration at the particle surface, and is the effective diffusivity. Diffusion is usually in the Knudsen regime when average pore radius is less than 100 nm.
For a given effectiveness factor,, and reaction order, n, the quantity is defined by the equation:
for small values of beta this can be approximated using the binomial theorem:
Assuming with a reaction order gives value of equal to 0.1. Therefore, for many conditions, if then pore diffusion limitations can be excluded.
The term file dynamics is the motion of many particles in a narrow channel.
A fractal catalytic model is a mathematical representation of chemical catalysis in an environment with fractal characteristics.
The Thiele modulus was developed by Ernest Thiele in his paper 'Relation between catalytic activity and size of particle' in 1939. Thiele reasoned that a large enough particle has a reaction rate so rapid that diffusion forces can only carry the product away from the surface of the catalyst particle. Therefore, only the surface of the catalyst would experience any reaction.
Decarboxylated and decarbonylated biofuels are renewable hydrocarbon fuels produced by converting biomass, by either decarboxylation or decarbonylation, into liquid transportation fuels, such as ethanol and biodiesel. Conversion of biomass to liquid fuels is preferred as an alternative to the extraction of fossil fuels because biomass removes carbon dioxide from the atmosphere as it grows through photosynthesis. When combusted, this carbon is re-released into the atmosphere, closing the carbon cycle and making biofuels carbon neutral under some conditions. First generation biofuels such as biodiesel are produced directly from crops, such as cereals, maize, sugar beet and cane, and rapeseed. Second generation fuels are produced from byproducts from production of food and other goods, as well as from household waste, used frying oil from restaurants, and slaughterhouse waste.
In electrochemistry, protein film voltammetry is a technique for examining the behavior of proteins immobilized on an electrode. The technique is applicable to proteins and enzymes that engage in electron transfer reactions and it is part of the methods available to study enzyme kinetics.

Heterogeneous gold catalysis refers to the use of elemental gold as a heterogeneous catalyst. As in most heterogeneous catalysis, the metal is typically supported on metal oxide. Furthermore, as seen in other heterogeneous catalysts, activity increases with a decreasing diameter of supported gold clusters. Several industrially relevant processes are also observed such as H2 activation, Water-gas shift reaction, and hydrogenation. One or two gold-catalyzed reactions may have been commercialized.
In chemistry, catalytic resonance theory was developed to describe the kinetics of reaction acceleration using dynamic catalyst surfaces. Catalytic reactions occurring on surfaces that undergo variation in surface binding energy and/or entropy exhibit overall increase in reaction rate when the surface binding energy frequencies are comparable to the natural frequencies of the surface reaction, adsorption, and desorption.
The Tammann temperature and the Hüttig temperature of a given material are approximations to the absolute temperatures at which atoms in a bulk crystal lattice (Tammann) or on the surface (Hüttig) of the solid material become sufficiently mobile to diffuse readily, and are consequently more chemically reactive and susceptible to recrystallization, agglomeration or sintering. These temperatures are equal to one-half (Tammann) or one-third (Hüttig) of the absolute temperature of the compound's melting point. The absolute temperatures are usually measured in Kelvin.















