
Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.

Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection period, people often have mild or no symptoms. Early symptoms can include fever, dark urine, abdominal pain, and yellow tinged skin. The virus persists in the liver, becoming chronic, in about 70% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.
Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV) co-infection is a multi-faceted, chronic condition that significantly impacts public health. According to the World Health Organization (WHO), 2 to 15% of those infected with HIV are also affected by HCV, increasing their risk of morbidity and mortality due to accelerated liver disease. The burden of co-infection is especially high in certain high-risk groups, such as intravenous drug users and men who have sex with men. These individuals who are HIV-positive are commonly co-infected with HCV due to shared routes of transmission including, but not limited to, exposure to HIV-positive blood, sexual intercourse, and passage of the Hepatitis C virus from mother to infant during childbirth.

Boceprevir is a protease inhibitor used to treat hepatitis caused by hepatitis C virus (HCV) genotype 1. It binds to the HCV nonstructural protein 3 active site.

Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without trans-membrane helices.

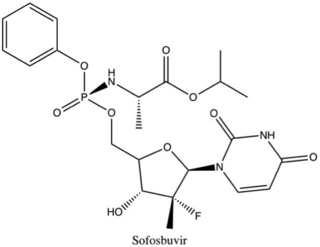
Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken by mouth.
Daclatasvir, sold under the brand name Daklinza, is an antiviral medication used in combination with other medications to treat hepatitis C (HCV). The other medications used in combination include sofosbuvir, ribavirin, and interferon, vary depending on the virus type and whether the person has cirrhosis. It is taken by mouth.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
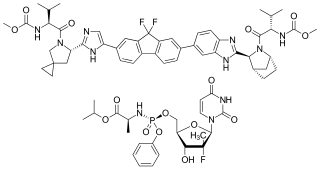
Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C. It is a fixed-dose combination of ledipasvir and sofosbuvir. Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1. Some evidence also supports use in HCV genotype 3 and 4. It is taken daily by mouth for 8–24 weeks.
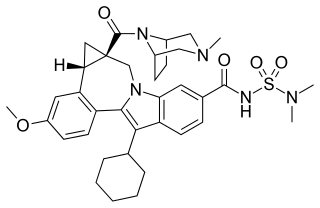
Beclabuvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection that has been studied in clinical trials. In February 2017, Bristol-Myers Squibb began sponsoring a post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function. From February 2014 to November 2016, a phase II clinical trial was conducted on the combination of asunaprevir/daclatasvir/beclabuvir on patients infected with both HIV and HCV. Furthermore, a recent meta-analysis of six published six clinical trials showed high response rates in HCV genotype 1-infected patients treated with daclatasvir, asunaprevir, and beclabuvir irrespective of ribavirin use, prior interferon-based therapy, or restriction on noncirrhotic patients, IL28B genotype, or baseline resistance-associated variants

Gamal Esmat is a professor at Endemic Medicine and Hepatogastroenterology Department, Cairo University. He was vice president of Cairo University for Graduate Studies and Research.
Anna Suk-Fong Lok is a gastroenterologist who studied in Hong Kong and moved to the United States in 1992. She is a Professor of Medicine at the University of Michigan in Ann Arbor and helped the World Health Organization (WHO) and American Association for the Study of Liver Diseases (AASLD) develop guidelines for medical professionals and recommendations for the general public on who should be treated and how treatments should be administered to persons with hepatitis B infections.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.
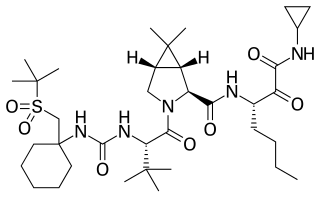
Narlaprevir, is an inhibitor of NS3/4A serine protease, intended for the treatment of chronic hepatitis C caused by genotype 1 virus in combination with other antiviral drugs.
Sofosbuvir/velpatasvir, sold under the brand name Epclusa among others, is a fixed-dose combination medication for the treatment of hepatitis C in adults. It combines sofosbuvir and velpatasvir. It is more than 90% effective for hepatitis C genotypes one through six. It also works for hepatitis C in those who also have cirrhosis or HIV/AIDS. It is taken by mouth.
Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.
The term Direct-acting antivirals (DAA) has long been associated with the combination of antiviral drugs used to treat hepatitis C infections. These are the more effective than older treatments such as ribavirin and interferon. The DAA drugs against hepatitis C are taken orally, as tablets, for 8 to 12 weeks. The treatment depends on the type or types (genotypes) of hepatitis C virus that are causing the infection. Both during and at the end of treatment, blood tests are used to monitor the effectiveness of the treatment and subsequent cure.

Interferon lambda 4 is one of the most recently discovered human genes and the newest addition to the interferon lambda protein family. This gene encodes the IFNL4 protein, which is involved in immune response to viral infection.

Annie F. Luetkemeyer is an American physician and researcher who is Professor of Medicine and Infectious Diseases at the University of California, San Francisco. She specializes in infectious diseases, in particular tuberculosis, human immunodeficiency virus and viral hepatitis. During the COVID-19 pandemic Luetkemeyer led a clinical trial of remdesivir. She has also researched treatment of COVID-19 as a co-infection with HIV.












