
Ceria-zirconia is a solid solution of cerium(IV) oxide (CeO2, also known as ceria) and zirconium oxide (ZrO2, also known as zirconia). [1]

Ceria-zirconia is a solid solution of cerium(IV) oxide (CeO2, also known as ceria) and zirconium oxide (ZrO2, also known as zirconia). [1]
The crystal structure adopted by ceria-zirconia depends on the Zr/Ce ratio and temperature. At very low Zr concentrations, ceria-zirconia exhibits the cubic fluorite structure, which is common to both pure ceria and cubic zirconia (pure zirconia normally only adopts a cubic structure at high temperatures). However, at higher Zr contents, other crystal structures are formed, including two different tetragonal phases at intermediate Zr concentrations, and a monoclinic phase at very high Zr concentrations. [2]
There is both experimental [3] [4] and theoretical [5] evidence showing that the decomposition of ceria-zirconia into Ce-rich and Zr-rich oxides is thermodynamically favorable in a wide range of solid solution compositions, indicating that ceria-zirconia is metastable with respect to phase separation.
Ceria-zirconia is widely used as a component in current three-way catalytic converters. [6] The ceria-based component of the converter has several functions, including promoting the dispersion of the noble metals in the catalyst, but also storing and releasing oxygen. [7] The incorporation of zirconium in modern converters, forming ceria-zirconia, improves the performance of the catalyst by enhancing the resistance of the material to sintering, and simultaneously increasing the ability of the oxide to accommodate oxygen vacancies in its structure. [6]

Zirconium is a chemical element; it has symbol Zr and atomic number 40. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role.

Cubic zirconia (abbreviated CZ) is the cubic crystalline form of zirconium dioxide (ZrO2). The synthesized material is hard and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate (ZrSiO4). It is sometimes erroneously called cubic zirconium.

Zirconium dioxide, sometimes known as zirconia, is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.
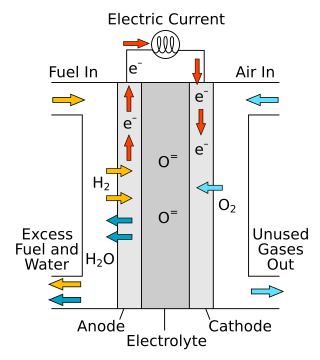
A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.

Zirconium tungstate (ZrWO4) is the zirconium salt of tungstic acid and has unusual properties. The phase formed at ambient pressure by reaction of ZrO2 and WO3 is a metastable cubic phase, which has negative thermal expansion characteristics, namely it shrinks over a wide range of temperatures when heated. In contrast to most other ceramics exhibiting negative CTE (coefficient of thermal expansion), the CTE of ZrW2O8 is isotropic and has a large negative magnitude (average CTE of -7.2x10−6K−1) over a wide range of temperature (-273 °C to 777 °C). A number of other phases are formed at high pressures.

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.

Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite, but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.
Zirconium hydride describes an alloy made by combining zirconium and hydrogen. Hydrogen acts as a hardening agent, preventing dislocations in the zirconium atom crystal lattice from sliding past one another. Varying the amount of hydrogen and the form of its presence in the zirconium hydride controls qualities such as the hardness, ductility, and tensile strength of the resulting zirconium hydride. Zirconium hydride with increased hydrogen content can be made harder and stronger than zirconium, but such zirconium hydride is also less ductile than zirconium.

A ceramic knife is a knife with a ceramic blade typically made from zirconium dioxide (ZrO2; also known as zirconia), rather than the steel used for most knives. Ceramic knife blades are usually produced through the dry-pressing and firing of powdered zirconia using solid-state sintering. The blades typically score 8.5 on the Mohs scale of mineral hardness, compared to 4.5 for normal steel and 7.5 to 8 for hardened steel and 10 for diamond. The resultant blade has a hard edge that stays sharp for much longer than conventional steel blades. However, the blade is brittle, subject to chipping, and will break rather than flex if twisted. The ceramic blade is sharpened by grinding the edges with a diamond-dust-coated grinding wheel.

Hafnium(IV) oxide is the inorganic compound with the formula HfO
2. Also known as hafnium dioxide or hafnia, this colourless solid is one of the most common and stable compounds of hafnium. It is an electrical insulator with a band gap of 5.3~5.7 eV. Hafnium dioxide is an intermediate in some processes that give hafnium metal.
Zirconium (IV) hydroxide, often called hydrous zirconia is an ill-defined material or family of materials variously described as ZrO2·nH2O and Zr(OH)4·nH2O. All are white solids with low solubility in water. These materials are widely employed in the preparation of solid acid catalysts.

Yttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" (ZrO2) and "yttria" (Y2O3), hence the name.

A solid oxide electrolyzer cell (SOEC) is a solid oxide fuel cell that runs in regenerative mode to achieve the electrolysis of water by using a solid oxide, or ceramic, electrolyte to produce hydrogen gas and oxygen. The production of pure hydrogen is compelling because it is a clean fuel that can be stored, making it a potential alternative to batteries, methane, and other energy sources. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to thermochemical and photocatalytic methods.
Transition metal oxides are compounds composed of oxygen atoms bound to transition metals. They are commonly utilized for their catalytic activity and semiconducting properties. Transition metal oxides are also frequently used as pigments in paints and plastics, most notably titanium dioxide. Transition metal oxides have a wide variety of surface structures which affect the surface energy of these compounds and influence their chemical properties. The relative acidity and basicity of the atoms present on the surface of metal oxides are also affected by the coordination of the metal cation and oxygen anion, which alter the catalytic properties of these compounds. For this reason, structural defects in transition metal oxides greatly influence their catalytic properties. The acidic and basic sites on the surface of metal oxides are commonly characterized via infrared spectroscopy, calorimetry among other techniques. Transition metal oxides can also undergo photo-assisted adsorption and desorption that alter their electrical conductivity. One of the more researched properties of these compounds is their response to electromagnetic radiation, which makes them useful catalysts for redox reactions, isotope exchange and specialized surfaces.
Gadolinium-doped ceria (GDC) (known alternatively as gadolinia-doped ceria, gadolinium-doped cerium oxide (GCO), cerium-gadolinium oxide (CGO), or cerium(IV) oxide, gadolinium-doped, formula Gd:CeO2) is a ceramic electrolyte used in solid oxide fuel cells (SOFCs). It has a cubic structure and a density of around 7.2 g/cm3 in its oxidised form. It is one of a class of ceria-doped electrolytes with higher ionic conductivity and lower operating temperatures (<700 °C) than those of yttria-stabilized zirconia, the material most commonly used in SOFCs. Because YSZ requires operating temperatures of 800–1000 °C to achieve maximal ionic conductivity, the associated energy and costs make GDC a more optimal (even "irreplaceable", according to researchers from the Fraunhofer Society) material for commercially viable SOFCs.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.

Heterogeneous gold catalysis refers to the use of elemental gold as a heterogeneous catalyst. As in most heterogeneous catalysis, the metal is typically supported on metal oxide. Furthermore, as seen in other heterogeneous catalysts, activity increases with a decreasing diameter of supported gold clusters. Several industrially relevant processes are also observed such as H2 activation, Water-gas shift reaction, and hydrogenation. One or two gold-catalyzed reactions may have been commercialized.

In crystallography, an anti-structure is obtained from a salt structure by exchanging anion and cation positions.
Lithium lanthanum zirconium oxide (LLZO, Li7La3Zr2O12) or lithium lanthanum zirconate is a lithium-stuffed garnet material that is under investigation for its use in solid-state electrolytes in lithium-based battery technologies. LLZO has a high ionic conductivity and thermal and chemical stability against reactions with prospective electrode materials, mainly lithium metal, giving it an advantage for use as an electrolyte in solid-state batteries. LLZO exhibits favorable characteristics, including the accessibility of starting materials, cost-effectiveness, and straightforward preparation and densification processes. These attributes position this zirconium-containing lithium garnet as a promising solid electrolyte for all-solid-state lithium-ion rechargeable batteries.
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known.