
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanthanide, as it can be dented with a fingernail and easily cut with a knife. When oxidation is removed a shiny-white metal is visible. Europium was isolated in 1901 and is named after the continent of Europe. Being a typical member of the lanthanide series, europium usually assumes the oxidation state +3, but the oxidation state +2 is also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic as compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.

Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, the usual oxidation state is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity.
The lanthanide or lanthanoid series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals.

Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. It is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China, as is the case with many other rare-earth metals.

Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of only two radioactive elements that are followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3.

Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite.

Zircon is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is ZrSiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green.

The rare-earth elements (REE), also called the rare-earth metals or in context rare-earth oxides, and sometimes the lanthanides, are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes.

Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral:

The mineral bastnäsite (or bastnaesite) is one of a family of three carbonate-fluoride minerals, which includes bastnäsite-(Ce) with a formula of (Ce, La)CO3F, bastnäsite-(La) with a formula of (La, Ce)CO3F, and bastnäsite-(Y) with a formula of (Y, Ce)CO3F. Some of the bastnäsites contain OH− instead of F− and receive the name of hydroxylbastnasite. Most bastnäsite is bastnäsite-(Ce), and cerium is by far the most common of the rare earths in this class of minerals. Bastnäsite and the phosphate mineral monazite are the two largest sources of cerium and other rare-earth elements.

Praseodymium is a chemical element with the symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered to be one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.

The europium anomaly is the phenomenon whereby the europium (Eu) concentration in a mineral is either enriched or depleted relative to some standard, commonly a chondrite or mid-ocean ridge basalt (MORB). In geochemistry a europium anomaly is said to be "positive" if the Eu concentration in the mineral is enriched relative to the other rare-earth elements (REEs), and is said to be "negative" if Eu is depleted relative to the other REEs.
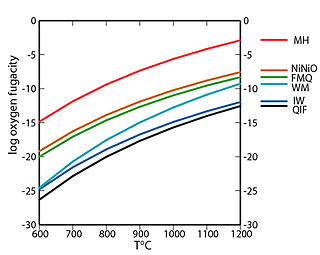
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals, and is never found in nature as a free element. 89Y is the only stable isotope, and the only isotope found in the Earth's crust.

Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure.
The Mountain Pass Mine, owned by MP Materials, is an open-pit mine of rare-earth elements on the south flank of the Clark Mountain Range in California, 53 miles (85 km) southwest of Las Vegas, Nevada. In 2020 the mine supplied 15.8% of the world's rare-earth production. It is the only rare-earth mining and processing facility in the United States.
Cerimetry or cerimetric titration, also known as cerate oximetry, is a method of volumetric chemical analysis developed by Ion Atanasiu. It is a redox titration in which an iron(II)–1,10-phenanthroline complex (ferroin) color change indicates the end point. Ferroin can be reversibly discolored in its oxidized form upon titration with a Ce4+ solution. The use of cerium(IV) salts as reagents for volumetric analysis was first proposed in the middle of 19th century, but systematic studies did not start until about 70 years later. Standard solutions can be prepared from different Ce4+ salts, but often cerium sulfate is chosen.

A pyrosilicate is a type of chemical compound; either an ionic compound that contains the pyrosilicate anionSi
2O6−
7, or an organic compound with the hexavalent ≡O
3Si-O-SiO
3≡ group. The anion is also called disilicate or diorthosilicate.
Cerium compounds are compounds containing the element cerium (Ce), a lanthanide. Cerium exists in two main oxidation states, Ce(III) and Ce(IV). This pair of adjacent oxidation states dominates several aspects of the chemistry of this element. Cerium(IV) aqueous solutions may be prepared by reacting cerium(III) solutions with the strong oxidizing agents peroxodisulfate or bismuthate. The value of E⦵(Ce4+/Ce3+) varies widely depending on conditions due to the relative ease of complexation and hydrolysis with various anions, although +1.72 V is representative. Cerium is the only lanthanide which has important aqueous and coordination chemistry in the +4 oxidation state.
















