Related Research Articles

The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are bonded together in different manners. For example, the allotropes of carbon include diamond, graphite, graphene, and fullerenes.

Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases, that arise from electromagnetic forces between atoms and electrons. More generally, the subject deals with condensed phases of matter: systems of many constituents with strong interactions among them. More exotic condensed phases include the superconducting phase exhibited by certain materials at extremely low cryogenic temperatures, the ferromagnetic and antiferromagnetic phases of spins on crystal lattices of atoms, the Bose–Einstein condensates found in ultracold atomic systems, and liquid crystals. Condensed matter physicists seek to understand the behavior of these phases by experiments to measure various material properties, and by applying the physical laws of quantum mechanics, electromagnetism, statistical mechanics, and other physics theories to develop mathematical models and predict the properties of extremely large groups of atoms.

A carbonate is a salt of carbonic acid, H2CO3, characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate groupO=C(−O−)2.

Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.

Sodium is a chemical element; it has symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans.

In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

Tantalum is a chemical element; it has symbol Ta and atomic number 73. Previously known as tantalium, it is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite and coltan.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates and Fermionic condensates, neutron-degenerate matter, and quark–gluon plasma. For a list of exotic states of matter, see the article List of states of matter.
Magma is the molten or semi-molten natural material from which all igneous rocks are formed. Magma is found beneath the surface of the Earth, and evidence of magmatism has also been discovered on other terrestrial planets and some natural satellites. Besides molten rock, magma may also contain suspended crystals and gas bubbles.
An electrolyte is a medium containing ions that are electrically conductive through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.
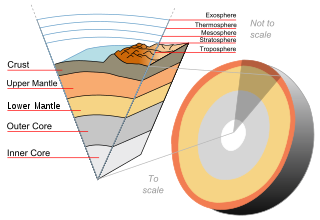
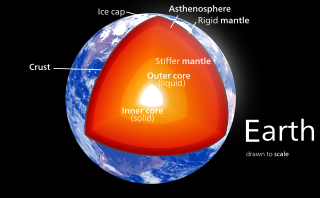
In planetary science, planetary differentiation is the process by which the chemical elements of a planetary body accumulate in different areas of that body, due to their physical or chemical behavior. The process of planetary differentiation is mediated by partial melting with heat from radioactive isotope decay and planetary accretion. Planetary differentiation has occurred on planets, dwarf planets, the asteroid 4 Vesta, and natural satellites.
A solid solution, a term popularly used for metals, is a homogeneous mixture of two different kinds of atoms in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions – solvents and solutes, depending on the relative abundance of the atomic species.

Molten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehiclesand for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.
Molten salt is salt which is solid at standard temperature and pressure but liquified due to elevated temperature. A salt that is liquid even at standard temperature and pressure is usually called a room-temperature ionic liquid, and molten salts are technically a class of ionic liquids.

A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. It is one of the four fundamental states of matter, and is the only state with a definite volume but no fixed shape.
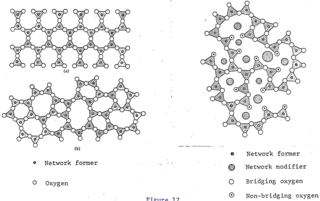
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.

FLiBe is the name of a molten salt made from a mixture of lithium fluoride (LiF) and beryllium fluoride. It is both a nuclear reactor coolant and solvent for fertile or fissile material. It served both purposes in the Molten-Salt Reactor Experiment (MSRE) at the Oak Ridge National Laboratory.

Magma oceans are vast fields of surface magma that exist during periods of a planet's or some natural satellite's accretion when the celestial body is completely or partly molten.
References
- 1 2 Starr, Michelle (2019-04-09). "A New 'State' of Matter Can Be Solid And Liquid at The Same Time". ScienceAlert. Retrieved 2023-10-31.
- ↑ Talukdar, Sandipan (2019-04-20). "Chain-Melted State: The Strange State of Matter". NewsClick.in. Retrieved 2023-10-31.
- 1 2 3 Mann, Adam (8 April 2019). "Confirmed: New phase of matter is solid and liquid at same time". National Geographic Society . Archived from the original on April 14, 2021. Retrieved 2023-10-31.
- ↑ McBride, E. E.; Munro, K. A.; Stinton, G. W.; Husband, R. J.; Briggs, R.; Liermann, H.-P.; McMahon, M. I. (2015-04-22). "One-dimensional chain melting in incommensurate potassium". Physical Review B. 91 (14): 144111. arXiv: 1504.02895 . Bibcode:2015PhRvB..91n4111M. doi:10.1103/PhysRevB.91.144111. S2CID 4989803.
- ↑ Naden Robinson, Victor; Zong, Hongxiang; Ackland, Graeme J.; Woolman, Gavin; Hermann, Andreas (2019-05-21). "On the chain-melted phase of matter". Proceedings of the National Academy of Sciences. 116 (21): 10297–10302. Bibcode:2019PNAS..11610297N. doi: 10.1073/pnas.1900985116 . ISSN 0027-8424. PMC 6535020 . PMID 30975752.
- ↑ "Elements can be solid and liquid at the same time". The University of Edinburgh. 2019-12-18. Retrieved 2023-10-31.
- 1 2 Duan, Daniel (2019-04-11). "New State of Matter: Liquid? Solid? Both!". Labroots.com. Retrieved 2023-11-03.
- ↑ "Elements can be solid and liquid at the same time, study reveals". EurekAlert!. Retrieved 2023-10-31.
- ↑ McKirdy, Euan (2019-04-09). "Researchers discover state of matter which is simultaneously solid and liquid". CNN. Retrieved 2023-10-31.
- ↑ Zong, Hongxiang; Robinson, Victor Naden; Hermann, Andreas; et al. (2021). "Free electron to electride transition in dense liquid potassium" (PDF). Nature Physics. 17 (8): 955–960. Bibcode:2021NatPh..17..955Z. doi:10.1038/s41567-021-01244-w. S2CID 236584774.
- ↑ Wang, Yong; Wang, Junjie; Hermann, Andreas; Liu, Cong; Gao, Hao; Tosatti, Erio; Wang, Hui-Tian; Xing, Dingyu; Sun, Jian (2021-01-11). "Electronically Driven 1D Cooperative Diffusion in a Simple Cubic Crystal". Physical Review X. 11 (1): 011006. Bibcode:2021PhRvX..11a1006W. doi: 10.1103/PhysRevX.11.011006 . hdl: 20.500.11820/dd6263d0-92f8-49da-bff6-9e08d035664a . ISSN 2160-3308. S2CID 234287896.