
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.

In modern physics, antimatter is defined as matter composed of the antiparticles of the corresponding particles in "ordinary" matter, and can be thought of as matter with reversed charge, parity, and time, known as CPT reversal. Antimatter occurs in natural processes like cosmic ray collisions and some types of radioactive decay, but only a tiny fraction of these have successfully been bound together in experiments to form antiatoms. Minuscule numbers of antiparticles can be generated at particle accelerators; however, total artificial production has been only a few nanograms. No macroscopic amount of antimatter has ever been assembled due to the extreme cost and difficulty of production and handling. Nonetheless, antimatter is an essential component of widely available applications related to beta decay, such as positron emission tomography, radiation therapy, and industrial imaging.

The electron is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.

Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be positive or negative. Like charges repel each other and unlike charges attract each other. An object with no net charge is referred to as electrically neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects.

In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. The Standard Model presently recognizes seventeen distinct particles—twelve fermions and five bosons. As a consequence of flavor and color combinations and antimatter, the fermions and bosons are known to have 48 and 13 variations, respectively. Among the 61 elementary particles embraced by the Standard Model number: electrons and other leptons, quarks, and the fundamental bosons. Subatomic particles such as protons or neutrons, which contain two or more elementary particles, are known as composite particles.

A muon is an elementary particle similar to the electron, with an electric charge of −1 e and spin-1/2, but with a much greater mass. It is classified as a lepton. As with other leptons, the muon is not thought to be composed of any simpler particles.

Particle physics or high-energy physics is the study of fundamental particles and forces that constitute matter and radiation. The field also studies combinations of elementary particles up to the scale of protons and neutrons, while the study of combination of protons and neutrons is called nuclear physics.
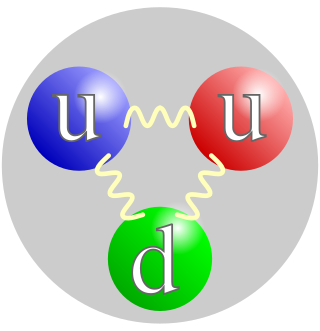
A proton is a stable subatomic particle, symbol
p
, H+, or 1H+ with a positive electric charge of +1 e (elementary charge). Its mass is slightly less than the mass of a neutron and approximately 1836 times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with a mass of approximately one atomic mass unit, are jointly referred to as nucleons (particles present in atomic nuclei).
Particle radiation is the radiation of energy by means of fast-moving subatomic particles. Particle radiation is referred to as a particle beam if the particles are all moving in the same direction, similar to a light beam.

In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.

In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles, or an elementary particle, which is not composed of other particles. Particle physics and nuclear physics study these particles and how they interact. Most force-carrying particles like photons or gluons are called bosons and, although they have quanta of energy, do not have rest mass or discrete diameters and are unlike the former particles that have rest mass and cannot overlap or combine which are called fermions. The W and Z bosons, however, are an exception to this rule and have relatively large rest masses at approximately 80GeV and 90GeV respectively.
In solid state physics, a charge carrier is a particle or quasiparticle that is free to move, carrying an electric charge, especially the particles that carry electric charges in electrical conductors. Examples are electrons, ions and holes. In a conducting medium, an electric field can exert force on these free particles, causing a net motion of the particles through the medium; this is what constitutes an electric current. The electron and the proton are the elementary charge carriers, each carrying one elementary charge (e), of the same magnitude and opposite sign.
Photoinduced charge separation is the process of an electron in an atom or molecule, being excited to a higher energy level by the absorption of a photon and then leaving the atom or molecule to free space, or to a nearby electron acceptor.

An ion is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.

The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.
The rms charge radius is a measure of the size of an atomic nucleus, particularly the proton distribution. The proton radius is about one femtometre = 10−15 metre. It can be measured by the scattering of electrons by the nucleus. Relative changes in the mean squared nuclear charge distribution can be precisely measured with atomic spectroscopy.
This glossary of physics is a list of definitions of terms and concepts relevant to physics, its sub-disciplines, and related fields, including mechanics, materials science, nuclear physics, particle physics, and thermodynamics. For more inclusive glossaries concerning related fields of science and technology, see Glossary of chemistry terms, Glossary of astronomy, Glossary of areas of mathematics, and Glossary of engineering.

The idea that matter consists of smaller particles and that there exists a limited number of sorts of primary, smallest particles in nature has existed in natural philosophy at least since the 6th century BC. Such ideas gained physical credibility beginning in the 19th century, but the concept of "elementary particle" underwent some changes in its meaning: notably, modern physics no longer deems elementary particles indestructible. Even elementary particles can decay or collide destructively; they can cease to exist and create (other) particles in result.










