Related Research Articles
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production. Coenzyme A consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid (B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
In polymer science, the polymer chain or simply backbone of a polymer is the main chain of a polymer. Polymers are often classified according to the elements in the main chains. The character of the backbone, i.e. its flexibility, determines the properties of the polymer. For example, in polysiloxanes (silicone), the backbone chain is very flexible, which results in a very low glass transition temperature of −123 °C. The polymers with rigid backbones are prone to crystallization in thin films and in solution. Crystallization in its turn affects the optical properties of the polymers, its optical band gap and electronic levels.
In chemistry, hydroxylation can refer to:
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein.

Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge. The Ser residue is generally in the sequence -Ser-Gly-X-Gly-, although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue.

Thiamine pyrophosphate (TPP or ThPP), or thiamine diphosphate (ThDP), or cocarboxylase is a thiamine (vitamin B1) derivative which is produced by the enzyme thiamine diphosphokinase. Thiamine pyrophosphate is a cofactor that is present in all living systems, in which it catalyzes several biochemical reactions.
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a larger hydrocarbon backbone. It is one factor in determining a molecule's properties and reactivity. A side chain is also known as a pendant chain, but a pendant group has a different definition.
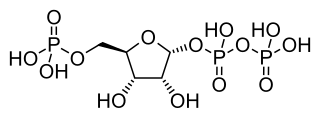
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate and a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase:
Oxidative decarboxylation reactions are oxidation reactions in which a carboxylate group is removed, forming carbon dioxide. They often occur in biological systems: there are many examples in the citric acid cycle. This type of reaction probably started early at the origin of life.
Palmitoyl-CoA is an acyl-CoA thioester. It is an "activated" form of palmitic acid and can be transported into the mitochondrial matrix by the carnitine shuttle system, and once inside can participate in beta-oxidation. Alternatively, palmitoyl-CoA is used as a substrate in the biosynthesis of sphingosine.
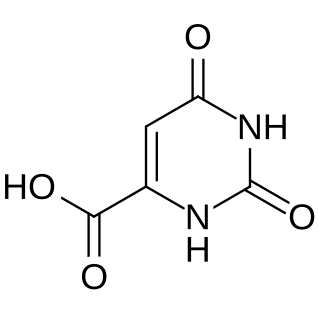
Orotic aciduria is a disease caused by an enzyme deficiency resulting in a decreased ability to synthesize pyrimidines. It was the first described enzyme deficiency of the de novo pyrimidine synthesis pathway.
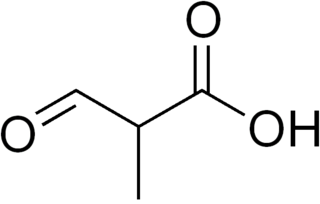
2-Methyl-3-oxopropanoic acid is an intermediate in the metabolism of thymine and valine.
Donald Herman Voet is an emeritus associate professor of chemistry at the University of Pennsylvania. His laboratory uses x-ray crystallography to understand structure-function relationships in proteins. He and his wife, Judith G. Voet, are authors of biochemistry text books that are widely used in undergraduate and graduate curricula.
Judith Greenwald Voet is a James Hammons Professor, Emerita in the Department of Chemistry and Biochemistry at Swarthmore College. Her research interests include enzyme reaction mechanisms and enzyme inhibition. She and her husband, Donald Voet, are authors of biochemistry textbooks that are widely used in undergraduate and graduate curricula.
Annette Gabriele Beck-Sickinger is a German chemist and biologist. She has been a full professor of Biochemistry and Bioorganic Chemistry at the University of Leipzig since 1999.

NDUFA4, mitochondrial complex associated is a protein that in humans is encoded by the NDUFA4 gene. The NDUFA4 protein was first described to be a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. However, recent research has described NDUFA4 as a subunit of cytochrome c oxidase. Mutations in the NDUFA4 gene are associated with Leigh's syndrome.

NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 7 is an enzyme that in humans is encoded by the NDUFA7 gene. The NDUFA7 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain.

Fundamentals of Biochemistry: Life at the Molecular Level is a biochemistry textbook written by Donald Voet, Judith G. Voet and Charlotte W. Pratt. Published by John Wiley & Sons, it is a common undergraduate biochemistry textbook.
Mysore Ananthamurthy Viswamitra (1932–2001) was an Indian molecular biophysicist and crystallographer, known for his pioneering work on the X-Ray structural studies of DNA fragments and nucleotide coenzyme molecules. His work is reported to have assisted in the development of the concept of sequence-dependent oligonucleotide conformation. He was an INSA senior scientist and an MSIL chair professor of physics at the Indian Institute of Science and a visiting professor at the University of Cambridge.
References
- ↑ Buehler, Lukas K. (January 2, 2000). "Reviews of books by Donald Voet, Judith Voet". Lukas K. Buehler. Retrieved 18 January 2010.
- ↑ Wood, E.J. (1 October 1999). "Book review: Biochemistry in a nutshell - Fundamentals of Biochemistry by Donald Voet, Judith G. Voet and Charlotte W. Pratt". Trends in Biochemical Sciences. 24 (10): 409–410. doi:10.1016/S0968-0004(99)01447-4.[ dead link ]
- ↑ Fundamentals of Biochemistry: Life at the Molecular Level. John Wiley & Sons. 2016. ISBN 978-1118918401.
- 1 2 "Charlotte Pratt - Associate Professor of Biology". Seattle Pacific University . Retrieved 5 August 2016.
- ↑ Pratt, Charlotte; Cornely, Kathleen (2004). Essential Biochemistry (Wiley internat. ed.). Hoboken, NJ: John Wiley & Sons. ISBN 9780471393870.
- ↑ "Speaker encourages students to ride bicycles". The Falcon. Retrieved 5 August 2016.