Related Research Articles

William Nunn Lipscomb Jr. was a Nobel Prize-winning American inorganic and organic chemist working in nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry.

Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH+
3 form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC.
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production. Coenzyme A consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid (B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
Anabolism is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-down aspect. Anabolism is usually synonymous with biosynthesis.
In chemistry, hydroxylation can refer to:
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic form. Half of the optically active substance becomes its mirror image (enantiomer) referred as racemic mixtures. If the racemization results in a mixture where the D and L enantiomers are present in equal quantities, the resulting sample is described as a racemic mixture or a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects.

Thiamine pyrophosphate (TPP or ThPP), or thiamine diphosphate (ThDP), or cocarboxylase is a thiamine (vitamin B1) derivative which is produced by the enzyme thiamine diphosphokinase. Thiamine pyrophosphate is a cofactor that is present in all living systems, in which it catalyzes several biochemical reactions.
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a larger hydrocarbon backbone. It is one factor in determining a molecule's properties and reactivity. A side chain is also known as a pendant chain, but a pendant group has a different definition.
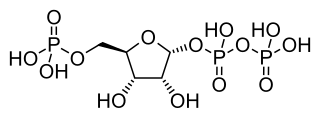
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate and a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase:

Enzyme catalysis is the increase in the rate of a process by a biological molecule, an "enzyme". Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs at a localized site, called the active site.
Palmitoyl-CoA is an acyl-CoA thioester. It is an "activated" form of palmitic acid and can be transported into the mitochondrial matrix by the carnitine shuttle system, and once inside can participate in beta-oxidation. Alternatively, palmitoyl-CoA is used as a substrate in the biosynthesis of sphingosine.
Judith Greenwald Voet is a James Hammons Professor, Emerita in the Department of Chemistry and Biochemistry at Swarthmore College. Her research interests include enzyme reaction mechanisms and enzyme inhibition. She and her husband, Donald Voet, are authors of biochemistry textbooks that are widely used in undergraduate and graduate curricula.
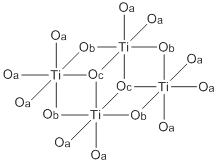
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a colorless liquid that is soluble in organic solvents but hydrolyzes readily. It is sold commercially as a colorless solution. Alkoxides of titanium(IV) and zirconium(IV) are used in organic synthesis and materials science. They adopt more complex structures than suggested by their empirical formulas.
Annette Gabriele Beck-Sickinger is a German chemist and biologist. She has been a full professor of Biochemistry and Bioorganic Chemistry at the University of Leipzig since 1999.

NDUFA4, mitochondrial complex associated is a protein that in humans is encoded by the NDUFA4 gene. The NDUFA4 protein was first described to be a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. However, recent research has described NDUFA4 as a subunit of cytochrome c oxidase. Mutations in the NDUFA4 gene are associated with Leigh's syndrome.
Diphosphorus tetroxide, or phosphorus tetroxide is an inorganic compound of phosphorus and oxygen. It has the empirical chemical formula P2O4. Solid phosphorus tetroxide (also referred to as phosphorus(III,V)-oxide) consists of variable mixtures of the mixed-valence oxides P4O7, P4O8 and P4O9.

Fundamentals of Biochemistry: Life at the Molecular Level is a biochemistry textbook written by Donald Voet, Judith G. Voet and Charlotte W. Pratt. Published by John Wiley & Sons, it is a common undergraduate biochemistry textbook.
Charlotte W. Pratt is an American biochemist and author. She is the co-author with Judith G. Voet and Donald Voet of the popular standard biochemistry textbook Fundamentals of Biochemistry.
Mysore Ananthamurthy Viswamitra (1932–2001) was an Indian molecular biophysicist and crystallographer, known for his pioneering work on the X-Ray structural studies of DNA fragments and nucleotide coenzyme molecules. His work is reported to have assisted in the development of the concept of sequence-dependent oligonucleotide conformation. He was an INSA senior scientist and an MSIL chair professor of physics at the Indian Institute of Science and a visiting professor at the University of Cambridge.
References
- ↑ Milner, Dorothy L; Committee On Chemists With Disabilities, American Chemical Society (2001). Teaching chemistry to students with disabilities: A manual for high schools, colleges, and graduate programs. ISBN 978-0-8412-3817-6.
- 1 2 3 "U. Penn, Dept. of Chemistry: Faculty Donald Voet". University of Pennsylvania. 2013. Retrieved 22 Aug 2013.
- ↑ Buehler, Lukas K. (January 2, 2000). "Reviews of books by Donald Voet, Judith Voet". Lukas K. Buehler. Retrieved 18 January 2010.
- 1 2 Wood, E.J. (1 October 1999). "Book review: Biochemistry in a nutshell - Fundamentals of Biochemistry by Donald Voet, Judith G. Voet and Charlotte W. Pratt". Trends in Biochemical Sciences. 24 (10): 409–410. doi:10.1016/S0968-0004(99)01447-4.
- ↑ Sodja, Ann (1996). "Book Review - Biochemistry". International Biodeterioration & Biodegradation. 37 (3–4): 233–235. doi:10.1016/0964-8305(96)88252-7.
- 1 2 3 "Biochemistry and Molecular Biology Education (BAMBED), a Journal for University, College, and High School Educators by Judith G. Voet1 and Donald H. Voet2, BAMBED Co-Editors-in-Chief" (PDF). Protein Databank Newsletter. RCSB PDB (38): 5. Summer 2008.
- ↑ Voet, D. and Lipscomb, W. N., "Molecular Structure of Carboranes. A 1,2-Dicarbaclovododecaborane Derivative, B10H10(CCH2Br)2," Inorg. Chem. 3, 1679 (1964).
- ↑ Voet, D. and Lipscomb, W. N., "Molecular and Crystal Structure of B7C2H11(CH3)2," Inorg. Chem. 6, 113-119 (1967).