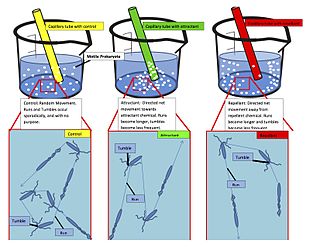
Chemotaxis is the movement of an organism or entity in response to a chemical stimulus. Somatic cells, bacteria, and other single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food by swimming toward the highest concentration of food molecules, or to flee from poisons. In multicellular organisms, chemotaxis is critical to early development and development as well as in normal function and health. In addition, it has been recognized that mechanisms that allow chemotaxis in animals can be subverted during cancer metastasis. The aberrant chemotaxis of leukocytes and lymphocytes also contribute to inflammatory diseases such as atherosclerosis, asthma, and arthritis. Sub-cellular components, such as the polarity patch generated by mating yeast, may also display chemotactic behavior.

The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.

Inflammation is part of the biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response involving immune cells, blood vessels, and molecular mediators. The function of inflammation is to eliminate the initial cause of cell injury, clear out damaged cells and tissues, and initiate tissue repair.

Cytokines are a broad and loose category of small proteins important in cell signaling. Due to their size, cytokines cannot cross the lipid bilayer of cells to enter the cytoplasm and therefore typically exert their functions by interacting with specific cytokine receptors on the target cell surface. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents.

Chemokines, or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In addition to playing a major role in the activation of host immune responses, chemokines are important for biological processes, including morphogenesis and wound healing, as well as in the pathogenesis of diseases like cancers.
Haptotaxis is the directional motility or outgrowth of cells, e.g. in the case of axonal outgrowth, usually up a gradient of cellular adhesion sites or substrate-bound chemoattractants. These gradients are naturally present in the extracellular matrix (ECM) of the body during processes such as angiogenesis or artificially present in biomaterials where gradients are established by altering the concentration of adhesion sites on a polymer substrate.

C-X-C chemokine receptor type 4 (CXCR-4) also known as fusin or CD184 is a protein that in humans is encoded by the CXCR4 gene. The protein is a CXC chemokine receptor.

Interleukin 8 is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage vesicles, the Weibel-Palade bodies. In humans, the interleukin-8 protein is encoded by the CXCL8 gene. IL-8 is initially produced as a precursor peptide of 99 amino acids which then undergoes cleavage to create several active IL-8 isoforms. In culture, a 72 amino acid peptide is the major form secreted by macrophages.
L-selectin, also known as CD62L, is a cell adhesion molecule found on the cell surface of leukocytes, and the blastocyst. It is coded for in the human by the SELL gene. L-selectin belongs to the selectin family of proteins, which recognize sialylated carbohydrate groups containing a Sialyl LewisX (sLeX) determinant. L-selectin plays an important role in both the innate and adaptive immune responses by facilitating leukocyte-endothelial cell adhesion events. These tethering interactions are essential for the trafficking of monocytes and neutrophils into inflamed tissue as well as the homing of lymphocytes to secondary lymphoid organs. L-selectin is also expressed by lymphoid primed hematopoietic stem cells and may participate in the migration of these stem cells to the primary lymphoid organs. In addition to its function in the immune response, L-selectin is expressed on embryonic cells and facilitates the attachment of the blastocyst to the endometrial endothelium during human embryo implantation.
Chemokine ligands 4 previously known as macrophage inflammatory protein (MIP-1β), is a protein which in humans is encoded by the CCL4 gene. CCL4 belongs to a cluster of genes located on 17q11-q21 of the chromosomal region. Identification and localization of the gene on the chromosome 17 was in 1990 although the discovery of MIP-1 was initiated in 1988 with the purification of a protein doublet corresponding to inflammatory activity from supernatant of endotoxin-stimulated murine macrophages. At that time, it was also named as "macrophage inflammatory protein-1" (MIP-1) due to its inflammatory properties.
Chemokine ligand 1 (CCL1) is also known as small inducible cytokine A1 and I-309 in humans. CCL1 is a small glycoprotein that belongs to the CC chemokine family.

Chemokine ligand 7 (CCL7) is a small cytokine that was previously called monocyte-chemotactic protein 3 (MCP3). CCL7 is a small protein that belongs to the CC chemokine family and is most closely related to CCL2.

Chemokine ligand 21 (CCL21) is a small cytokine belonging to the CC chemokine family. This chemokine is also known as 6Ckine, exodus-2, and secondary lymphoid-tissue chemokine (SLC). CCL21 elicits its effects by binding to a cell surface chemokine receptor known as CCR7. The main function of CCL21 is to guide CCR7 expressing leukocytes to the secondary lymphoid organs, such as lymph nodes and Peyer´s patches.

Chemokine ligand 9 (CXCL9) is a small cytokine belonging to the CXC chemokine family that is also known as monokine induced by gamma interferon (MIG). The CXCL9 is one of the chemokine which plays role to induce chemotaxis, promote differentiation and multiplication of leukocytes, and cause tissue extravasation.

The chemokine ligand 1 (CXCL1) is a small peptide belonging to the CXC chemokine family that acts as a chemoattractant for several immune cells, especially neutrophils or other non-hematopoietic cells to the site of injury or infection and plays an important role in regulation of immune and inflammatory responses. It was previously called GRO1 oncogene, GROα, neutrophil-activating protein 3 (NAP-3) and melanoma growth stimulating activity, alpha (MGSA-α). CXCL1 was first cloned from a cDNA library of genes induced by platelet-derived growth factor (PDGF) stimulation of BALB/c-3T3 murine embryonic fibroblasts and named "KC" for its location in the nitrocellulose colony hybridization assay. This designation is sometimes erroneously believed to be an acronym and defined as "keratinocytes-derived chemokine". Rat CXCL1 was first reported when NRK-52E cells were stimulated with interleukin-1β (IL-1β) and lipopolysaccharide (LPS) to generate a cytokine that was chemotactic for rat neutrophils, cytokine-induced neutrophil chemoattractant (CINC). In humans, this protein is encoded by the gene Cxcl1 and is located on human chromosome 4 among genes for other CXC chemokines.
C-X-C motif chemokine 5 is a protein that in humans is encoded by the CXCL5 gene.

CD137, a member of the tumor necrosis factor (TNF) receptor family, is a type 1 transmembrane protein, expressed on surfaces of leukocytes and non-immune cells. Its alternative names are tumor necrosis factor receptor superfamily member 9 (TNFRSF9), 4-1BB, and induced by lymphocyte activation (ILA). It is of interest to immunologists as a co-stimulatory immune checkpoint molecule, and as a potential target in cancer immunotherapy.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

C-C chemokine receptor type 7 is a protein that in humans is encoded by the CCR7 gene. Two ligands have been identified for this receptor: the chemokines ligand 19 (CCL19/ELC) and ligand 21 (CCL21). The ligands have similar affinity for the receptor, though CCL19 has been shown to induce internalisation of CCR7 and desensitisation of the cell to CCL19/CCL21 signals. CCR7 is a transmembrane protein with 7 transmembrane domains, which is coupled with heterotrimeric G proteins, which transduce the signal downstream through various signalling cascades. The main function of the receptor is to guide immune cells to immune organs by detecting specific chemokines, which these tissues secrete.

Dedicator of cytokinesis protein 2 (Dock2) is a protein encoded in the human by the DOCK2 gene. Dock2 is a large protein involved in intracellular signalling networks. It is a member of the DOCK-A subfamily of the DOCK family of guanine nucleotide exchange factors (GEFs) which function as activators of small G-proteins. Dock2 specifically activates isoforms of the small G protein Rac.



















