This article needs additional citations for verification .(May 2021) |
Chimeric nucleases are an example of engineered proteins which must comprise a DNA-binding domain to give sequence specificity and a nuclease domain for DNA cleavage.
This article needs additional citations for verification .(May 2021) |
Chimeric nucleases are an example of engineered proteins which must comprise a DNA-binding domain to give sequence specificity and a nuclease domain for DNA cleavage.
DNA-binding domains including the basic helix-loop-helix, zinc finger, helix-turn-helix and leucine zipper motifs have been used in construction of sequence-specific nucleases. Of these, zinc fingers have been suggested the most important due to their modularity, allowing construction of a tailor-made DNA-binding domain. [1]
The nuclease domain is responsible for physical cleavage of DNA strands and may introduce either single stranded or double-stranded breaks. FokI is an example of a sequence-specific endonuclease whose non-specific nuclease domain introduces double stranded breaks and has been used in a variety of experiments including identification of high- and low-affinity binding sites of transcription factors in vitro, to study recruitment of factors to promoter sites in vivo using protein position identification with a nuclease tail assay and to study proteins specific to interaction with DNA in the Z-DNA conformation (Durai et al., 2005 and references therein).
A restriction enzyme, restriction endonuclease, REase, ENase orrestrictase is an enzyme that cleaves DNA into fragments at or near specific recognition sites within molecules known as restriction sites. Restriction enzymes are one class of the broader endonuclease group of enzymes. Restriction enzymes are commonly classified into five types, which differ in their structure and whether they cut their DNA substrate at their recognition site, or if the recognition and cleavage sites are separate from one another. To cut DNA, all restriction enzymes make two incisions, once through each sugar-phosphate backbone of the DNA double helix.
Gene knockouts are a widely used genetic engineering technique that involves the targeted removal or inactivation of a specific gene within an organism's genome. This can be done through a variety of methods, including homologous recombination, CRISPR-Cas9, and TALENs.
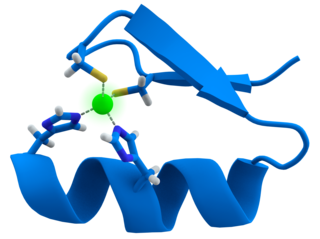
A zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) which stabilizes the fold. It was originally coined to describe the finger-like appearance of a hypothesized structure from the African clawed frog (Xenopus laevis) transcription factor IIIA. However, it has been found to encompass a wide variety of differing protein structures in eukaryotic cells. Xenopus laevis TFIIIA was originally demonstrated to contain zinc and require the metal for function in 1983, the first such reported zinc requirement for a gene regulatory protein followed soon thereafter by the Krüppel factor in Drosophila. It often appears as a metal-binding domain in multi-domain proteins.
Gene knockdown is an experimental technique by which the expression of one or more of an organism's genes is reduced. The reduction can occur either through genetic modification or by treatment with a reagent such as a short DNA or RNA oligonucleotide that has a sequence complementary to either gene or an mRNA transcript.

DNA-binding proteins are proteins that have DNA-binding domains and thus have a specific or general affinity for single- or double-stranded DNA. Sequence-specific DNA-binding proteins generally interact with the major groove of B-DNA, because it exposes more functional groups that identify a base pair.
A DNA-binding domain (DBD) is an independently folded protein domain that contains at least one structural motif that recognizes double- or single-stranded DNA. A DBD can recognize a specific DNA sequence or have a general affinity to DNA. Some DNA-binding domains may also include nucleic acids in their folded structure.
Therapeutic gene modulation refers to the practice of altering the expression of a gene at one of various stages, with a view to alleviate some form of ailment. It differs from gene therapy in that gene modulation seeks to alter the expression of an endogenous gene whereas gene therapy concerns the introduction of a gene whose product aids the recipient directly.
Zinc-finger nucleases (ZFNs) are artificial restriction enzymes generated by fusing a zinc finger DNA-binding domain to a DNA-cleavage domain. Zinc finger domains can be engineered to target specific desired DNA sequences and this enables zinc-finger nucleases to target unique sequences within complex genomes. By taking advantage of endogenous DNA repair machinery, these reagents can be used to precisely alter the genomes of higher organisms. Alongside CRISPR/Cas9 and TALEN, ZFN is a prominent tool in the field of genome editing.

The restriction endonuclease Fok1, naturally found in Flavobacterium okeanokoites, is a bacterial type IIS restriction endonuclease consisting of an N-terminal DNA-binding domain and a non sequence-specific DNA cleavage domain at the C-terminal. Once the protein is bound to duplex DNA via its DNA-binding domain at the 5'-GGATG-3' recognition site, the DNA cleavage domain is activated and cleaves the DNA at two locations, regardless of the nucleotide sequence at the cut site. The DNA is cut 9 nucleotides downstream of the motif on the forward strand, and 13 nucleotides downstream of the motif on the reverse strand, producing two sticky ends with 4-bp overhangs.

Nuclease S1 is an endonuclease enzyme that splits single-stranded DNA (ssDNA) and RNA into oligo- or mononucleotides. This enzyme catalyses the following chemical reaction
Zinc finger protein chimera are chimeric proteins composed of a DNA-binding zinc finger protein domain and another domain through which the protein exerts its effect. The effector domain may be a transcriptional activator (A) or repressor (R), a methylation domain (M) or a nuclease (N).
Meganucleases are endodeoxyribonucleases characterized by a large recognition site ; as a result this site generally occurs only once in any given genome. For example, the 18-base pair sequence recognized by the I-SceI meganuclease would on average require a genome twenty times the size of the human genome to be found once by chance. Meganucleases are therefore considered to be the most specific naturally occurring restriction enzymes.
TALeffectors are proteins secreted by some β- and γ-proteobacteria. Most of these are Xanthomonads. Plant pathogenic Xanthomonas bacteria are especially known for TALEs, produced via their type III secretion system. These proteins can bind promoter sequences in the host plant and activate the expression of plant genes that aid bacterial infection. The TALE domain responsible for binding to DNA is known to have 1.5 to 33.5 short sequences that are repeated multiple times. Each of these repeats was found to be specific for a certain base pair of the DNA. These repeats also have repeat variable residues (RVD) that can detect specific DNA base pairs. They recognize plant DNA sequences through a central repeat domain consisting of a variable number of ~34 amino acid repeats. There appears to be a one-to-one correspondence between the identity of two critical amino acids in each repeat and each DNA base in the target sequence. These proteins are interesting to researchers both for their role in disease of important crop species and the relative ease of retargeting them to bind new DNA sequences. Similar proteins can be found in the pathogenic bacterium Ralstonia solanacearum and Burkholderia rhizoxinica, as well as yet unidentified marine microorganisms. The term TALE-likes is used to refer to the putative protein family encompassing the TALEs and these related proteins.
Recombinant adeno-associated virus (rAAV) based genome engineering is a genome editing platform centered on the use of recombinant AAV vectors that enables insertion, deletion or substitution of DNA sequences into the genomes of live mammalian cells. The technique builds on Mario Capecchi and Oliver Smithies' Nobel Prize–winning discovery that homologous recombination (HR), a natural hi-fidelity DNA repair mechanism, can be harnessed to perform precise genome alterations in mice. rAAV mediated genome-editing improves the efficiency of this technique to permit genome engineering in any pre-established and differentiated human cell line, which, in contrast to mouse ES cells, have low rates of HR.

Transcription activator-like effector nucleases (TALEN) are restriction enzymes that can be engineered to cut specific sequences of DNA. They are made by fusing a TAL effector DNA-binding domain to a DNA cleavage domain. Transcription activator-like effectors (TALEs) can be engineered to bind to practically any desired DNA sequence, so when combined with a nuclease, DNA can be cut at specific locations. The restriction enzymes can be introduced into cells, for use in gene editing or for genome editing in situ, a technique known as genome editing with engineered nucleases. Alongside zinc finger nucleases and CRISPR/Cas9, TALEN is a prominent tool in the field of genome editing.
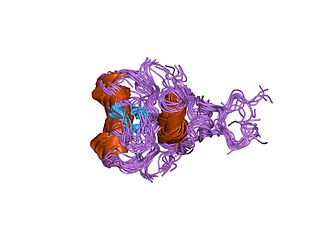
In molecular biology, GATA zinc fingers are zinc-containing domains found in a number of transcription factors. Some members of this class of zinc fingers specifically bind the DNA sequence (A/T)GATA(A/G) in the regulatory regions of genes., giving rise to the name of the domain. In these domains, a single zinc ion is coordinated by 4 cysteine residues. NMR studies have shown the core of the Znf to comprise 2 irregular anti-parallel beta-sheets and an alpha-helix, followed by a long loop to the C-terminal end of the finger. The N-terminal part, which includes the helix, is similar in structure, but not sequence, to the N-terminal zinc module of the glucocorticoid receptor DNA-binding domain. The helix and the loop connecting the 2 beta-sheets interact with the major groove of the DNA, while the C-terminal tail wraps around into the minor groove. Interactions between the Znf and DNA are mainly hydrophobic, explaining the preponderance of thymines in the binding site; a large number of interactions with the phosphate backbone have also been observed. Two GATA zinc fingers are found in GATA-family transcription factors. However, there are several proteins that only contain a single copy of the domain. It is also worth noting that many GATA-type Znfs have not been experimentally demonstrated to be DNA-binding domains. Furthermore, several GATA-type Znfs have been demonstrated to act as protein-recognition domains. For example, the N-terminal Znf of GATA1 binds specifically to a zinc finger from the transcriptional coregulator FOG1 (ZFPM1).

Genome editing, or genome engineering, or gene editing, is a type of genetic engineering in which DNA is inserted, deleted, modified or replaced in the genome of a living organism. Unlike early genetic engineering techniques that randomly inserts genetic material into a host genome, genome editing targets the insertions to site-specific locations. The basic mechanism involved in genetic manipulations through programmable nucleases is the recognition of target genomic loci and binding of effector DNA-binding domain (DBD), double-strand breaks (DSBs) in target DNA by the restriction endonucleases, and the repair of DSBs through homology-directed recombination (HDR) or non-homologous end joining (NHEJ).

Genetic engineering techniques allow the modification of animal and plant genomes. Techniques have been devised to insert, delete, and modify DNA at multiple levels, ranging from a specific base pair in a specific gene to entire genes. There are a number of steps that are followed before a genetically modified organism (GMO) is created. Genetic engineers must first choose what gene they wish to insert, modify, or delete. The gene must then be isolated and incorporated, along with other genetic elements, into a suitable vector. This vector is then used to insert the gene into the host genome, creating a transgenic or edited organism.

Cas9 is a 160 kilodalton protein which plays a vital role in the immunological defense of certain bacteria against DNA viruses and plasmids, and is heavily utilized in genetic engineering applications. Its main function is to cut DNA and thereby alter a cell's genome. The CRISPR-Cas9 genome editing technique was a significant contributor to the Nobel Prize in Chemistry in 2020 being awarded to Emmanuelle Charpentier and Jennifer Doudna.
Since antiretroviral therapy requires a lifelong treatment regimen, research to find more permanent cures for HIV infection is currently underway. It is possible to synthesize zinc finger nucleotides with zinc finger components that selectively bind to specific portions of DNA. Conceptually, targeting and editing could focus on host cellular co-receptors for HIV or on proviral HIV DNA.