Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in reinforcing the DNA during cell division, preventing DNA damage, and regulating gene expression and DNA replication. During mitosis and meiosis, chromatin facilitates proper segregation of the chromosomes in anaphase; the characteristic shapes of chromosomes visible during this stage are the result of DNA being coiled into highly condensed chromatin.

In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei and in most Archaeal phyla. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30-nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 mm) of 30 nm diameter chromatin fibers.

A nucleosome is the basic structural unit of DNA packaging in eukaryotes. The structure of a nucleosome consists of a segment of DNA wound around eight histone proteins and resembles thread wrapped around a spool. The nucleosome is the fundamental subunit of chromatin. Each nucleosome is composed of a little less than two turns of DNA wrapped around a set of eight proteins called histones, which are known as a histone octamer. Each histone octamer is composed of two copies each of the histone proteins H2A, H2B, H3, and H4.
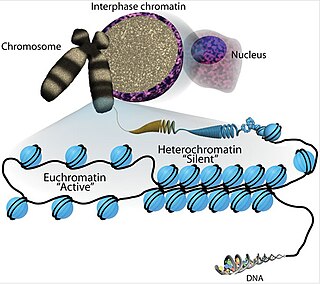
Euchromatin is a lightly packed form of chromatin that is enriched in genes, and is often under active transcription. Euchromatin stands in contrast to heterochromatin, which is tightly packed and less accessible for transcription. 92% of the human genome is euchromatic.

Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression.

In molecular biology, a histone octamer is the eight-protein complex found at the center of a nucleosome core particle. It consists of two copies of each of the four core histone proteins. The octamer assembles when a tetramer, containing two copies of H3 and two of H4, complexes with two H2A/H2B dimers. Each histone has both an N-terminal tail and a C-terminal histone-fold. Each of these key components interacts with DNA in its own way through a series of weak interactions, including hydrogen bonds and salt bridges. These interactions keep the DNA and the histone octamer loosely associated, and ultimately allow the two to re-position or to separate entirely.
HMGN proteins are members of the broader class of high mobility group (HMG) chromosomal proteins that are involved in regulation of transcription, replication, recombination, and DNA repair.
Histone H1 is one of the five main histone protein families which are components of chromatin in eukaryotic cells. Though highly conserved, it is nevertheless the most variable histone in sequence across species.

Histone H2A is one of the five main histone proteins involved in the structure of chromatin in eukaryotic cells.

The solenoid structure of chromatin is a model for the structure of the 30 nm fibre. It is a secondary chromatin structure which helps to package eukaryotic DNA into the nucleus.
Chromatin remodeling is the dynamic modification of chromatin architecture to allow access of condensed genomic DNA to the regulatory transcription machinery proteins, and thereby control gene expression. Such remodeling is principally carried out by 1) covalent histone modifications by specific enzymes, e.g., histone acetyltransferases (HATs), deacetylases, methyltransferases, and kinases, and 2) ATP-dependent chromatin remodeling complexes which either move, eject or restructure nucleosomes. Besides actively regulating gene expression, dynamic remodeling of chromatin imparts an epigenetic regulatory role in several key biological processes, egg cells DNA replication and repair; apoptosis; chromosome segregation as well as development and pluripotency. Aberrations in chromatin remodeling proteins are found to be associated with human diseases, including cancer. Targeting chromatin remodeling pathways is currently evolving as a major therapeutic strategy in the treatment of several cancers.

Histone H2A.Z is a protein that in humans is encoded by the H2AZ1 gene.
Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Nucleosomes consist of approximately 146 bp of DNA wrapped around a histone octamer composed of pairs of each of the four core histones. The chromatin fiber is further compacted through the interaction of a linker histone, H1, with the DNA between the nucleosomes to form higher order chromatin structures. The H2AFZ gene encodes a replication-independent member of the histone H2A family that is distinct from other members of the family. Studies in mice have shown that this particular histone is required for embryonic development and indicate that lack of functional histone H2A leads to embryonic lethality.

Histone H3.1 is a protein in humans that is encoded by the H3C1 gene.

Histone H3.1 is a protein that in humans is encoded by the HIST1H3I gene.
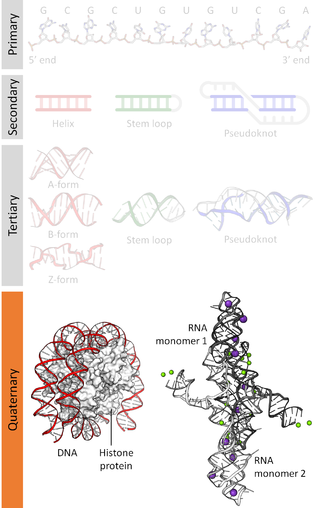
Nucleic acidquaternary structure refers to the interactions between separate nucleic acid molecules, or between nucleic acid molecules and proteins. The concept is analogous to protein quaternary structure, but as the analogy is not perfect, the term is used to refer to a number of different concepts in nucleic acids and is less commonly encountered. Similarly other biomolecules such as proteins, nucleic acids have four levels of structural arrangement: primary, secondary, tertiary, and quaternary structure. Primary structure is the linear sequence of nucleotides, secondary structure involves small local folding motifs, and tertiary structure is the 3D folded shape of nucleic acid molecule. In general, quaternary structure refers to 3D interactions between multiple subunits. In the case of nucleic acids, quaternary structure refers to interactions between multiple nucleic acid molecules or between nucleic acids and proteins. Nucleic acid quaternary structure is important for understanding DNA, RNA, and gene expression because quaternary structure can impact function. For example, when DNA is packed into heterochromatin, therefore exhibiting a type of quaternary structure, gene transcription will be inhibited.

H2A histone family, member B3 is a protein that in humans is encoded by the H2AFB3 gene.
Pioneer factors are transcription factors that can directly bind condensed chromatin. They can have positive and negative effects on transcription and are important in recruiting other transcription factors and histone modification enzymes as well as controlling DNA methylation. They were first discovered in 2002 as factors capable of binding to target sites on nucleosomal DNA in compacted chromatin and endowing competency for gene activity during hepatogenesis. Pioneer factors are involved in initiating cell differentiation and activation of cell-specific genes. This property is observed in histone fold-domain containing transcription factors and other transcription factors that use zinc finger(s) for DNA binding.

In molecular biology, the linker histone H1 is a protein family forming a critical component of eukaryotic chromatin. H1 histones bind to the linker DNA exiting from the nucleosome core particle, while the core histones form the octamer core of the nucleosome around which the DNA is wrapped.

MNase-seq, short for micrococcal nuclease digestion with deep sequencing, is a molecular biological technique that was first pioneered in 2006 to measure nucleosome occupancy in the C. elegans genome, and was subsequently applied to the human genome in 2008. Though, the term ‘MNase-seq’ had not been coined until a year later, in 2009. Briefly, this technique relies on the use of the non-specific endo-exonuclease micrococcal nuclease, an enzyme derived from the bacteria Staphylococcus aureus, to bind and cleave protein-unbound regions of DNA on chromatin. DNA bound to histones or other chromatin-bound proteins may remain undigested. The uncut DNA is then purified from the proteins and sequenced through one or more of the various Next-Generation sequencing methods.
Chromodomain helicase DNA-binding (CHD) proteins is a subfamily of ATP-dependent chromatin remodeling complexes (remodelers). All remodelers fall under the umbrella of RNA/DNA helicase superfamily 2. In yeast, CHD complexes are primarily responsible for nucleosome assembly and organization. These complexes play an additional role in multicellular eukaryotes, assisting in chromatin access and nucleosome editing.














