A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions), which results in a compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

Clathrate hydrates, or gas hydrates, clathrates, or hydrates, are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded, frozen water molecules. In other words, clathrate hydrates are clathrate compounds in which the host molecule is water and the guest molecule is typically a gas or liquid. Without the support of the trapped molecules, the lattice structure of hydrate clathrates would collapse into conventional ice crystal structure or liquid water. Most low molecular weight gases, including O2, H2, N2, CO2, CH4, H2S, Ar, Kr, Xe, and Cl2 as well as some higher hydrocarbons and freons, will form hydrates at suitable temperatures and pressures. Clathrate hydrates are not officially chemical compounds, as the enclathrated guest molecules are never bonded to the lattice. The formation and decomposition of clathrate hydrates are first order phase transitions, not chemical reactions. Their detailed formation and decomposition mechanisms on a molecular level are still not well understood. Clathrate hydrates were first documented in 1810 by Sir Humphry Davy who found that water was a primary component of what was earlier thought to be solidified chlorine.
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.
Thermoelectric materials show the thermoelectric effect in a strong or convenient form.

In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".

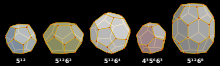
Skutterudite is a cobalt arsenide mineral containing variable amounts of nickel and iron substituting for cobalt with the ideal formula CoAs3. Some references give the arsenic a variable formula subscript of 2–3. High nickel varieties are referred to as nickel-skutterudite, previously chloanthite. It is a hydrothermal ore mineral found in moderate to high temperature veins with other Ni-Co minerals. Associated minerals are arsenopyrite, native silver, erythrite, annabergite, nickeline, cobaltite, silver sulfosalts, native bismuth, calcite, siderite, barite and quartz. It is mined as an ore of cobalt and nickel with a by-product of arsenic.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.
In chemistry, bond energy (BE) is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy for all bonds of the same type within the same chemical species.
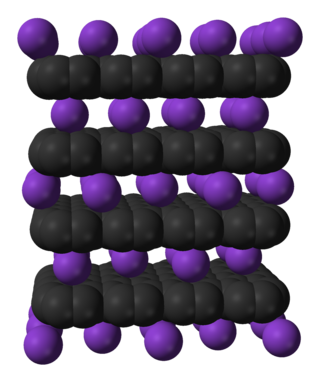
Intercalation is the reversible inclusion or insertion of a molecule into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides.

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another.
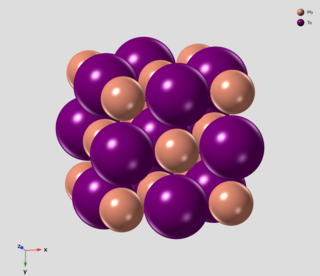
Lead telluride is a compound of lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.32 eV. It occurs naturally as the mineral altaite.

A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2, or 3 dimensions.

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices.
A Bjerrum defect is a crystallographic defect which is specific to ice, and which is partly responsible for the electrical properties of ice. It was first proposed by Niels Bjerrum in 1952 in order to explain the electrical polarization of ice in an electric field. A hydrogen bond normally has one proton, but a hydrogen bond with a Bjerrum defect will have either two protons or no proton. D-defects are more energetically favorable than L-defects. The unfavorable defect strain is resolved when a water molecule pivots about an oxygen atom to produce hydrogen bonds with single protons. Dislocations of ice Ih along a slip plane create pairs of Bjerrum defects, one D defect and one L defect.

The hydration number of a compound is defined as the number of molecules of water bonded to a central ion, often a metal cation. The hydration number is related to the broader concept of solvation number, the number of solvent molecules bonded to a central atom. The hydration number varies with the atom or ion of interest.

Solid nitrogen is a number of solid forms of the element nitrogen, first observed in 1884. Solid nitrogen is mainly the subject of academic research, but low-temperature, low-pressure solid nitrogen is a substantial component of bodies in the outer Solar System and high-temperature, high-pressure solid nitrogen is a powerful explosive, with higher energy density than any other non-nuclear material.
In inorganic chemistry, Hofmann clathrates refers to materials with the formula Ni(CN)2(NH3)(C6H6). These materials are a type of coordination polymer that have properties of inclusion compounds. They have attracted attention because they can be used to separate xylenes. On a conceptual level, Hofmann clathrates can be viewed as forerunners to metal-organic frameworks (MOFs).
Arsenide iodides or iodide arsenides are compounds containing anions composed of iodide (I−) and arsenide (As3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the arsenide chlorides, arsenide bromides, phosphide iodides, and antimonide iodides.