
Arsenopyrite is an iron arsenic sulfide (FeAsS). It is a hard metallic, opaque, steel grey to silver white mineral with a relatively high specific gravity of 6.1.

Mimetite is a lead arsenate chloride mineral (Pb5(AsO4)3Cl) which forms as a secondary mineral in lead deposits, usually by the oxidation of galena and arsenopyrite. The name derives from the Greek Μιμητής mimetes, meaning "imitator" and refers to mimetite's resemblance to the mineral pyromorphite. This resemblance is not coincidental, as mimetite forms a mineral series with pyromorphite (Pb5(PO4)3Cl) and with vanadinite (Pb5(VO4)3Cl). Notable occurrences are Mapimi, Durango, Mexico and Tsumeb, Namibia.

Adamite is a zinc arsenate hydroxide mineral, Zn2AsO4OH. It is a mineral that typically occurs in the oxidized or weathered zone above zinc ore occurrences. Pure adamite is colorless, but usually it possess yellow color due to Fe compounds admixture. Tints of green also occur and are connected with copper substitutions in the mineral structure. Olivenite is a copper arsenate that is isostructural with adamite and there is considerable substitution between zinc and copper resulting in an intermediate called cuproadamite. Zincolivenite is a recently discovered mineral being an intermediate mineral with formula CuZn(AsO4)(OH). Manganese, cobalt, and nickel also substitute in the structure. An analogous zinc phosphate, tarbuttite, is known.

Olivenite is a copper arsenate mineral, formula Cu2AsO4OH. It crystallizes in the monoclinic system (pseudo-orthorhombic), and is sometimes found in small brilliant crystals of simple prismatic habit terminated by domal faces. More commonly, it occurs as globular aggregates of acicular crystals, these fibrous forms often having a velvety luster; sometimes it is lamellar in structure, or soft and earthy.

Arsenic trioxide is an inorganic compound with the formula As
2O
3. As an industrial chemical, its major uses include the manufacture of wood preservatives, pesticides, and glass. It is sold under the brand name Trisenox among others when used as a medication to treat a type of cancer known as acute promyelocytic leukemia. For this use it is given by injection into a vein.

Hausmannite is a complex oxide, or a mixed oxide, of manganese containing both di- and tri-valent manganese. Its chemical formula can be represented as MnIIMnIII2O4, or more simply noted as MnO·Mn2O3, or Mn3O4, as commonly done for magnetite, the corresponding iron oxide. It belongs to the spinel group and forms tetragonal crystals. Hausmannite is a brown to black metallic mineral with Mohs hardness of 5.5 and a specific gravity of 4.8.
In chemistry, an arsenite is a chemical compound containing an arsenic oxyanion where arsenic has oxidation state +3. Note that in fields that commonly deal with groundwater chemistry, arsenite is used generically to identify soluble AsIII anions. IUPAC have recommended that arsenite compounds are to be named as arsenate(III), for example ortho-arsenite is called trioxidoarsenate(III). Ortho-arsenite contrasts to the corresponding anions of the lighter members of group 15, phosphite which has the structure HPO2−3 and nitrite, NO−2 which is bent.

Pharmacosiderite is a hydrated basic ferric arsenate, with the chemical formula KFe4(AsO4)3(OH)4·(6-7)H2O and a molecular weight of 873.38 g/mol. It consists of the elements arsenic, iron, hydrogen, potassium, sodium and oxygen. It has a Mohs hardness of 2 to 3, about that of a finger nail. Its specific gravity is about 2.7 to 2.9, has indistinct cleavage, and is usually transparent or translucent. It has a yellow or white streak and a yellow, green, brown or red color. Its lustre is adamantine, vitreous and resinous, and it has conchoidal, brittle and sectile fracture.
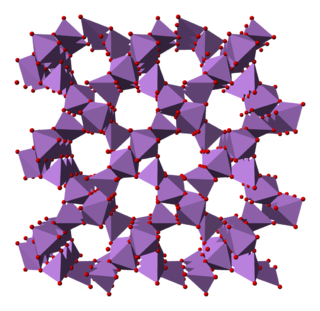
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

Jacobsite is a manganese iron oxide mineral. It is in the spinel group and forms a solid solution series with magnetite. The chemical formula is (Mn,Mg)Fe2O4 or with oxidation states and substitutions: (Mn2+,Fe2+,Mg)(Fe3+,Mn3+)2O4.

Arsenolite is an arsenic mineral, chemical formula As4O6. It is formed as an oxidation product of arsenic sulfides. Commonly found as small octahedra it is white, but impurities of realgar or orpiment may give it a pink or yellow hue. It can be associated with its dimorph claudetite (a monoclinic form of As2O3) as well as realgar (As4S4), orpiment (As2S3) and erythrite, Co3(AsO4)2·8H2O.

Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.
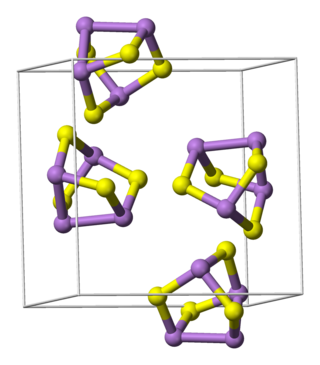
Pararealgar is an arsenic sulfide mineral with the chemical formula As4S4, also represented as AsS. It forms gradually from realgar under exposure to light. Its name derives from the fact that its elemental composition is identical to realgar, As4S4. It is soft with a Mohs hardness of 1 - 1.5, is yellow orange in colour, and its monoclinic prismatic crystals are very brittle, easily crumbling to powder.

Polydymite, Ni2+Ni23+S4, is a supergene thiospinel sulfide mineral associated with the weathering of primary pentlandite nickel sulfide.

Conichalcite, CaCu(AsO4)(OH), is a relatively common arsenate mineral related to duftite (PbCu(AsO4)(OH)). It is green, often botryoidal, and occurs in the oxidation zone of some metal deposits. It occurs with limonite, malachite, beudantite, adamite, cuproadamite, olivenite and smithsonite.

Picropharmacolite, Ca4Mg(AsO3OH)2(AsO4)2·11H2O, is a rare arsenate mineral. It was named in 1819 from the Greek for bitter, in allusion to its magnesium content, and its chemical similarity to pharmacolite. The mineral irhtemite, Ca4Mg(AsO3OH)2(AsO4)2·4H2O, has the same composition as picropharmacolite, except that it has only four water molecules per formula unit, instead of eleven. It may be formed by the dehydration of picropharmacolite.

Arseniosiderite is a rare arsenate mineral formed by the oxidation of other arsenic-containing minerals, such as scorodite or arsenopyrite. It occurs in association with beudantite, carminite, dussertite, pharmacolite, pitticite, adamite and erythrite. The name arseniosiderite reflects two major elements of the mineral, arsenic and iron.

Pharmacolite is an uncommon calcium arsenate mineral with formula CaHAsO4·2(H2O). It occurs as soft, white clusters of fibrous crystals and encrustations which crystallize in the monoclinic system. It is the arsenate analogue of the sulfate gypsum and the phosphate brushite.
Lucabindiite is a mineral discovered in 1998 from the La Fossa crater at Vulcano, the Aeolian islands off the coast of Italy. It has the chemical formula As4O6(Cl,Br) and is hexagonal. After months of collecting sublimates and encrustations, the researchers discovered lucabindiite which was found on the surface of pyroclastic breccia. The mineral is named after Luca Bindi, who was a professor of mineralogy and former head of the Division of Mineralogy of the Natural History Museum of the University of Florence.

Compounds of arsenic resemble in some respects those of phosphorus which occupies the same group (column) of the periodic table. The most common oxidation states for arsenic are: −3 in the arsenides, which are alloy-like intermetallic compounds, +3 in the arsenites, and +5 in the arsenates and most organoarsenic compounds. Arsenic also bonds readily to itself as seen in the square As3−
4 ions in the mineral skutterudite. In the +3 oxidation state, arsenic is typically pyramidal owing to the influence of the lone pair of electrons.


















