
Salvinorin A is the main active psychotropic molecule in Salvia divinorum. Salvinorin A is considered a dissociative hallucinogen.
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine.
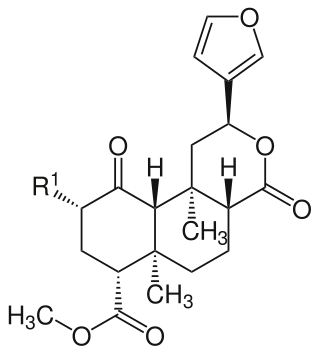
Salvinorins are a group of natural chemical compounds and their structural analogs. Several salvinorins have been isolated from Salvia divinorum. They are classified as diterpenoid furanolactones. Salvinorin A is a hallucinogen with dissociative effects.
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and anti-inflammatory.

Salvia glutinosa, the glutinous sage, sticky sage, Jupiter's sage, or Jupiter's distaff, is a herbaceous perennial plant belonging to the family Lamiaceae.

Decalin, a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives.

A furanolactone is a heterocyclic chemical compound that contains both lactone and furan rings in its chemical structure.
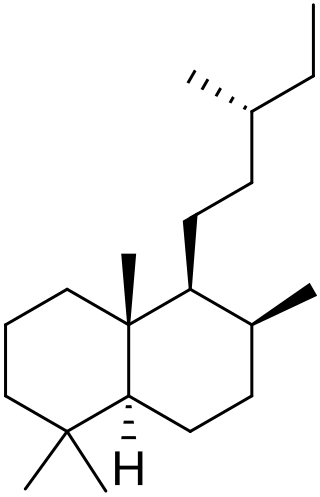
Labdane is a natural bicyclic diterpene. It forms the structural core for a wide variety of natural products collectively known as labdanes or labdane diterpenes. The labdanes were so named because the first members of the class were originally obtained from labdanum, a resin derived from the gum rockrose.
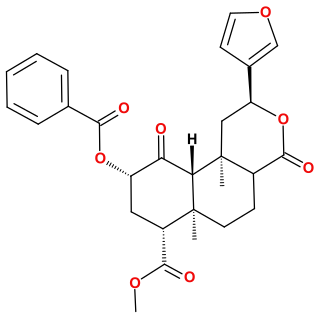
Herkinorin is an opioid analgesic that is an analogue of the natural product salvinorin A. It was discovered in 2005 during structure-activity relationship studies into neoclerodane diterpenes, the family of chemical compounds of which salvinorin A is a member.
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and a dienophile are both part of the same molecule. The reaction leads to the formation of the same cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system. This reaction gives rise to various natural derivatives of decalin.
The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.

Salvia divinorum is a plant species with transient psychoactive properties when its leaves, or extracts made from the leaves, are administered by smoking, chewing, or drinking. The leaves contain the potent compound salvinorin A and can induce a dissociative state and hallucinations.

Salvia polystachya is a herbaceous perennial native to central Mexico and south into Guatemala and Panama, typically growing at elevations from 5,000 to 10,000 feet in mild climates where there is some summer rain. It one of the species used as chia and it is rarely seen in horticulture.

Salvia recognita is a woody-based perennial that is endemic to central Turkey, typically growing in light shade at the base of cliffs, at elevations of less than 4,000 feet (1,200 m). This species has been reported to contain salvinorin A. However, this report has not been replicated, and a previous study of 441 Salvia species from many regions found salvinorin A only in Salvia divinorum, from Mexico.

Taxodone is a naturally occurring diterpenoid found in Taxodium distichum, Rosmarinus officinalis (rosemary), several salvia species and other plants, along with its oxidized rearrangement product, taxodione. Taxodone and taxodione exhibit anticancer, antibacterial, antioxidant, antifungal, insecticide, and antifeedant activities.
The legal status of Salvia divinorum in the United States varies, with 29 states having completely banned it and others considering proposals for banning its use.
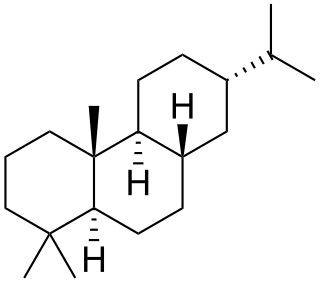
Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes.

Sugiol is a phenolic abietane derivative of ferruginol and can be used as a biomarker for specific families of conifers. The presence of sugiol can be used to identify the Cupressaceae s.1., podocarpaceae, and Araucaraiaceae families of conifers. The polar terpenoids are among the most resistant molecules to degradation besides n-alkanes and fatty acids, affording them high viability as biomarkers due to their longevity in the sedimentary record. Significant amounts of sugiol has been detected in fossil wood dated to the Eocene and Miocene periods, as well as a sample of Protopodocarpoxylon dated to the middle Jurassic.

Kurkinorin is a non-nitrogenous, extremely selective centrally acting μ-opioid receptor agonist derived from salvinorin A with no sedating or rewarding effects.














